
Binding selectivity
Encyclopedia
Binding selectivity refers to the differing affinities with which different ligand
s bind to a substrate forming a complex
. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry
and in chemical separation process
es.
. Then, the two interactions may be characterized by equilibrium constants KAB and KAC.The constant used here are association constants. Dissociation constants are used in some contexts. A dissociation constant is the reciprocal of an association constant.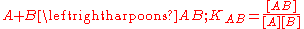
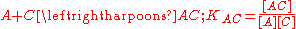
[..] represents a concentration
. A selectivity coefficient is defined as the ratio of the two equilibrium constants.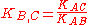
The selectivity coefficient is in fact the equilibrium constant for the displacement reaction
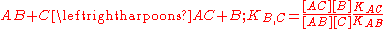
It is easy to show that the same definition applies to complexes of a different stoichiometry, ApBq and ApCq. The greater the selectivity coefficient, the more the ligand C will displace the ligand B from the complex formed with the substrate A. An alternative interpretation is that the greater the selectivity coefficient, the lower the concentration of C that is needed to displace B from AB. Selectivity coefficients are determined experimentally by measuring the two equilibrium constants, KAB and KAC.
molecule, embedded in either the plasma membrane or the cytoplasm
of a cell, to which one or more specific kinds of signalling molecules may bind. A ligand
may be a peptide
or another small molecule, such as a neurotransmitter
, a hormone
, a pharmaceutical drug, or a toxin. The specificity of a receptor is determined by its spatial geometry and the way it binds
to the ligand through non-covalent interactions
, such as hydrogen bond
ing or Van der Waals force
s.
If a receptor can be isolated a synthetic drug can be developed either to stimulate the receptor, an agonist
or to block it, an antagonist
. The stomach ulcer drug cimetidine
was developed as an H2 antagonist by chemically engineering the molecule for maximum specificity to an isolated tissue containing the receptor. The further use of quantitative structure-activity relationship
s (QSAR) led to the development of other agents such as ranitidine
.
, Pb2+ or mercury
, Hg2+ selectivity against calcium
, Ca2+ and magnesium
, Mg2+, is essential in order that the treatment does not remove essential metals.
Selectivity is determined by various factors. In the case of iron overload, which may occur in individuals with β-thalessemia who have received blood transfusion
s, the target metal ion is in the +3 oxidation state
and so forms stronger complexes than the divalent ions. It also forms stronger complexes with oxygen-donor ligands than with nitrogen-donor ligands. deferoxamine
, a naturally occurring siderophore
produced by the actinobacter Streptomyces pilosus and was used initially as a chelation therapy agent. Synthetic siderophores such as deferiprone
and deferasirox
have been developed, using the known structure of deferoxamine as a starting point. Chelation occurs with the two oxygen atoms.
Wilson's disease
is caused by a defect in copper
metabolism which results in accumulation of copper metal in various organs of the body. The target ion in this case is divalent, Cu2+. This ion is classified as borderline in the scheme of Ahrland, Chatt and Davies. This means that it forms roughly equally strong complexes with ligands whose donor atoms are N, O or F as with ligands whose donor atoms are P, S or Cl. Penicillamine
, which contains nitrogen and sulphur donor atoms, is used as this type of ligand binds more strongly to copper ions than to calcium and magnesium ions.
Treatment of poisoning by heavy metals such as lead and mercury is more problematical, because the ligands used do not have high specificity relative to calcium. For example, EDTA
may be administered as a calcium salt to reduce the removal of calcium from bone together with the heavy metal.
between the stationary phase and the mobile phase. The selectivity factor is equal to the selectivity coefficient with the added assumption that the activity
of the stationary phase, the substrate in this case, is equal to 1, the standard assumption for a pure phase. The resolution of a chromatographic column, RS is related to the selectivity factor by: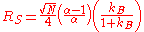
where α is selectivity factor, N is the number of theoretical plate
s kA and kB are the retention factors of the two analytes. Retention factors are proportional to distribution coefficients. In practice substances with a selectivity factor very close to 1 can be separated. This is particularly true in gas-liquid chromatography
where column lengths up to 60 m are possible, providing a very large number of theoretical plates.
In ion-exchange chromatography the selectivity coefficient is defined in a slightly different way
. In one process, the metal ions in aqueous solution are made to form complexes with tributylphosphate (TBP), which are extracted into an organic solvent such as kerosene
. Complete separation is effected by using a countercurrent exchange
method. A number of cells are arranged as a cascade
. After equilibration, the aqueous component of each cell is transferred to the previous cell and the organic component is transferred to the next cell, which initially contains only water. In this way the metal ion with the most stable complex passes down the cascade in the organic phase and the metal with the least stable complex passes up the cascade in the aqueous phase.
If solubility in the organic phase is not an issue, a selectivity coefficient is equal to the ratio of the stability constant
s of the TBP complexes of two metal ions. For lanthanoid elements which are adjacent in the periodic table
this ratio is not much greater than 1, so many cells are needed in the cascade.
distinguish one particular ion from others. The selectivity coefficient, KB,C is evaluated by means of the emf response of the ion-selective electrode in mixed solutions of the primary ion, B, and interfering ion, C (fixed interference method) or less desirably, in separate solutions of B and C (separate
solution method). For example, a potassium
ion-selective membrane electrode utilizes the naturally occurring macrocyclic antibiotic
valinomycin
. In this case the cavity in the macrocyclic ring is just the right size to encapsulate the potassium ion, but too large to bind the sodium ion, the most likely interference, strongly.
Chemical sensors are being developed for specific target molecules and ions in which the target (guest) form a complex with a sensor (host). The sensor is designed to be an excellent match in terms of the size and shape of the target in order to provide for the maximum binding selectivity. An indicator is associated with the sensor which undergoes a change when the target forms a complex with the sensor . The indicator change is usually a colour change (gray to yellow in the illustration) seen in absorbance or, with greater sensitivity, luminescence
. The indicator may be attached to the sensor via a spacer, in the ISR arrangement, or it may be displaced from the sensor, IDA arrangement.
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
s bind to a substrate forming a complex
Complex
A complex is a whole that comprehends a number of intricate parts, especially one with interconnected or mutually related parts; for example, a complex of buildings.Complex may refer to:-Biology:...
. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
and in chemical separation process
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
es.
Selectivity coefficient
The concept of selectivity is used to quantify the extent to which a given substrate, A, binds two different ligands, B and C. The simplest case is where the complexes formed have 1:1 stoichiometryStoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
. Then, the two interactions may be characterized by equilibrium constants KAB and KAC.The constant used here are association constants. Dissociation constants are used in some contexts. A dissociation constant is the reciprocal of an association constant.
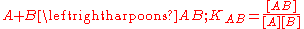
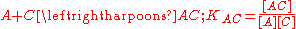
[..] represents a concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
. A selectivity coefficient is defined as the ratio of the two equilibrium constants.
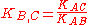
The selectivity coefficient is in fact the equilibrium constant for the displacement reaction
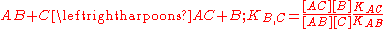
It is easy to show that the same definition applies to complexes of a different stoichiometry, ApBq and ApCq. The greater the selectivity coefficient, the more the ligand C will displace the ligand B from the complex formed with the substrate A. An alternative interpretation is that the greater the selectivity coefficient, the lower the concentration of C that is needed to displace B from AB. Selectivity coefficients are determined experimentally by measuring the two equilibrium constants, KAB and KAC.
Biochemistry
In biochemistry the substrate is known as a receptor. A receptor is a proteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
molecule, embedded in either the plasma membrane or the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
of a cell, to which one or more specific kinds of signalling molecules may bind. A ligand
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
may be a peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
or another small molecule, such as a neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
, a hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
, a pharmaceutical drug, or a toxin. The specificity of a receptor is determined by its spatial geometry and the way it binds
Binding (molecular)
Molecular binding is an attractive interaction between two molecules which results in a stable association in which the molecules are in close proximity to each other...
to the ligand through non-covalent interactions
Noncovalent bonding
A noncovalent bond is a type of chemical bond, typically between macromolecules, that does not involve the sharing of pairs of electrons, but rather involves more dispersed variations of electromagnetic interactions. The noncovalent bond is the dominant type of bond between supermolecules in...
, such as hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing or Van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
s.
If a receptor can be isolated a synthetic drug can be developed either to stimulate the receptor, an agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
or to block it, an antagonist
Antagonist
An antagonist is a character, group of characters, or institution, that represents the opposition against which the protagonist must contend...
. The stomach ulcer drug cimetidine
Cimetidine
Cimetidine INN is a histamine H2-receptor antagonist that inhibits the production of acid in the stomach. It is largely used in the treatment of heartburn and peptic ulcers. It is marketed by GlaxoSmithKline under the trade name Tagamet...
was developed as an H2 antagonist by chemically engineering the molecule for maximum specificity to an isolated tissue containing the receptor. The further use of quantitative structure-activity relationship
Quantitative structure-activity relationship
Quantitative structure–activity relationship or QSPR is the process by which chemical structure is quantitatively correlated with a well defined process, such as biological activity or chemical reactivity.For example, biological activity can be expressed quantitatively as the concentration of a...
s (QSAR) led to the development of other agents such as ranitidine
Ranitidine
Ranitidine is a histamine H2-receptor antagonist that inhibits stomach acid production. It is commonly used in treatment of peptic ulcer disease and gastroesophageal reflux disease . Ranitidine is also used alongside fexofenadine and other antihistamines for the treatment of skin conditions...
.
Chelation therapy
Chelation therapy is a form of medical treatment in which a chelating ligandThe term "ligand" here refers to binding to a metal. In the definition of selectivity coefficient this "ligand" is in fact the substrate and ligand in that definition is the metal ion. is used to selectively remove a metal from the body. When the metal exists as a divalent ion, such as with leadLead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, Pb2+ or mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, Hg2+ selectivity against calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, Ca2+ and magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, Mg2+, is essential in order that the treatment does not remove essential metals.
Selectivity is determined by various factors. In the case of iron overload, which may occur in individuals with β-thalessemia who have received blood transfusion
Blood transfusion
Blood transfusion is the process of receiving blood products into one's circulation intravenously. Transfusions are used in a variety of medical conditions to replace lost components of the blood...
s, the target metal ion is in the +3 oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
and so forms stronger complexes than the divalent ions. It also forms stronger complexes with oxygen-donor ligands than with nitrogen-donor ligands. deferoxamine
Deferoxamine
Deferoxamine is a bacterial siderophore produced by the actinobacteria Streptomyces pilosus. It has medical applications as a chelating agent used to remove excess iron from the body...
, a naturally occurring siderophore
Siderophore
Siderophores are small, high-affinity iron chelating compounds secreted by grasses and microorganisms such as bacteria and fungi...
produced by the actinobacter Streptomyces pilosus and was used initially as a chelation therapy agent. Synthetic siderophores such as deferiprone
Deferiprone
Deferiprone is an oral drug that chelates iron and is used to treat thalassaemia major.It has been licensed for use in Europe and Asia for many years while awaiting approval in Canada and the United States. On October 14, 2011, however, "the U.S...
and deferasirox
Deferasirox
Deferasirox is a rationally-designed oral iron chelator. Its main use is to reduce chronic iron overload in patients who are receiving long-term blood transfusions for conditions such as beta-thalassemia and other chronic anemias...
have been developed, using the known structure of deferoxamine as a starting point. Chelation occurs with the two oxygen atoms.
Wilson's disease
Wilson's disease
Wilson's disease or hepatolenticular degeneration is an autosomal recessive genetic disorder in which copper accumulates in tissues; this manifests as neurological or psychiatric symptoms and liver disease...
is caused by a defect in copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
metabolism which results in accumulation of copper metal in various organs of the body. The target ion in this case is divalent, Cu2+. This ion is classified as borderline in the scheme of Ahrland, Chatt and Davies. This means that it forms roughly equally strong complexes with ligands whose donor atoms are N, O or F as with ligands whose donor atoms are P, S or Cl. Penicillamine
Penicillamine
Penicillamine is a pharmaceutical of the chelator class. It is sold under the trade names of Cuprimine and Depen. The pharmaceutical form is D-penicillamine, as L-penicillamine is toxic...
, which contains nitrogen and sulphur donor atoms, is used as this type of ligand binds more strongly to copper ions than to calcium and magnesium ions.
Treatment of poisoning by heavy metals such as lead and mercury is more problematical, because the ligands used do not have high specificity relative to calcium. For example, EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
may be administered as a calcium salt to reduce the removal of calcium from bone together with the heavy metal.
Chromatography
In column chromatography a mixture of substances is dissolved in a mobile phase and passed over a stationary phase in a column. A selectivity factor is defined as the ratio of distribution coefficients, which describe the equilibrium distribution of an analyteAnalyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
between the stationary phase and the mobile phase. The selectivity factor is equal to the selectivity coefficient with the added assumption that the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the stationary phase, the substrate in this case, is equal to 1, the standard assumption for a pure phase. The resolution of a chromatographic column, RS is related to the selectivity factor by:
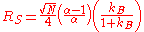
where α is selectivity factor, N is the number of theoretical plate
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
s kA and kB are the retention factors of the two analytes. Retention factors are proportional to distribution coefficients. In practice substances with a selectivity factor very close to 1 can be separated. This is particularly true in gas-liquid chromatography
Gas-liquid chromatography
Gas chromatography , is a common type of chromatography used in analytical chemistry for separating and analysing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture...
where column lengths up to 60 m are possible, providing a very large number of theoretical plates.
In ion-exchange chromatography the selectivity coefficient is defined in a slightly different way
Solvent extraction
Solvent extraction is use to extract individual lanthanoid elements from the mixtures found in nature in ores such as monaziteMonazite
Monazite is a reddish-brown phosphate mineral containing rare earth metals. It occurs usually in small isolated crystals. There are actually at least four different kinds of monazite, depending on relative elemental composition of the mineral:...
. In one process, the metal ions in aqueous solution are made to form complexes with tributylphosphate (TBP), which are extracted into an organic solvent such as kerosene
Kerosene
Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros...
. Complete separation is effected by using a countercurrent exchange
Countercurrent exchange
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or...
method. A number of cells are arranged as a cascade
Cascade (chemical engineering)
In chemical engineering, a cascade is a plant consisting of several similar stages with each processing the output from the previous stage. Cascades are most commonly used in isotope separation, distillation and other separation or purification processes....
. After equilibration, the aqueous component of each cell is transferred to the previous cell and the organic component is transferred to the next cell, which initially contains only water. In this way the metal ion with the most stable complex passes down the cascade in the organic phase and the metal with the least stable complex passes up the cascade in the aqueous phase.
If solubility in the organic phase is not an issue, a selectivity coefficient is equal to the ratio of the stability constant
Stability constant
Stability constant may refer to:*Equilibrium constant*Acid dissociation constant*Stability constants of complexes...
s of the TBP complexes of two metal ions. For lanthanoid elements which are adjacent in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
this ratio is not much greater than 1, so many cells are needed in the cascade.
Chemical sensors
A potentiometric selectivity coefficient defines the ability of an ion-selective electrode todistinguish one particular ion from others. The selectivity coefficient, KB,C is evaluated by means of the emf response of the ion-selective electrode in mixed solutions of the primary ion, B, and interfering ion, C (fixed interference method) or less desirably, in separate solutions of B and C (separate
solution method). For example, a potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
ion-selective membrane electrode utilizes the naturally occurring macrocyclic antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
valinomycin
Valinomycin
Valinomycin is a dodecadepsipeptide antibiotic.Valinomycin is obtained from the cells of several Streptomyces strains, among which "S. tsusimaensis" and S. fulvissimus....
. In this case the cavity in the macrocyclic ring is just the right size to encapsulate the potassium ion, but too large to bind the sodium ion, the most likely interference, strongly.
Chemical sensors are being developed for specific target molecules and ions in which the target (guest) form a complex with a sensor (host). The sensor is designed to be an excellent match in terms of the size and shape of the target in order to provide for the maximum binding selectivity. An indicator is associated with the sensor which undergoes a change when the target forms a complex with the sensor . The indicator change is usually a colour change (gray to yellow in the illustration) seen in absorbance or, with greater sensitivity, luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
. The indicator may be attached to the sensor via a spacer, in the ISR arrangement, or it may be displaced from the sensor, IDA arrangement.
See also
- BindingBinding (molecular)Molecular binding is an attractive interaction between two molecules which results in a stable association in which the molecules are in close proximity to each other...
- Affinity
- Functional selectivityFunctional SelectivityFunctional selectivity is the ligand-dependent selectivity for certain signal transduction pathways in one and the same receptor. This can be present when a receptor has several possible signal transduction pathways...

