Leucine-rich repeat
Encyclopedia
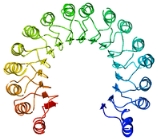
A leucine-rich repeat is a protein
structural motif
that forms an α/β horseshoe fold
. It is composed of repeating 20–30 amino acid
stretches that are unusually rich in the hydrophobic amino acid leucine
. Typically, each repeat unit has beta strand
-turn
-alpha helix
structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent
and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.
Leucine-rich repeats are frequently involved in the formation of protein–protein interactions.
, but other proteins such as the tropomyosin
regulator tropomodulin
and the toll-like receptor
also share the motif. In fact, the toll-like receptor
possesses 10 successive LRR motifs which serve to bind antigen.
Although the canonical LRR protein contains approximately one helix for every beta strand, variants that form beta-alpha superhelix folds sometimes have long loops rather than helices linking successive beta strands.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
structural motif
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
that forms an α/β horseshoe fold
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
. It is composed of repeating 20–30 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
stretches that are unusually rich in the hydrophobic amino acid leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
. Typically, each repeat unit has beta strand
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
-turn
Turn (biochemistry)
A turn is an element of secondary structure in proteins where the polypeptide chain reverses its overall direction.- Definition :According to the most common definition, a turn is a structural motif where the Cα atoms of two residues separated by few peptide bonds are in close approach A turn is...
-alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.
Leucine-rich repeats are frequently involved in the formation of protein–protein interactions.
Examples
Leucine-rich repeat motifs have been identified in a large number of functionally unrelated proteins. The best-known example is the ribonuclease inhibitorRibonuclease inhibitor
Ribonuclease inhibitor is a large , acidic , leucine-rich repeat protein that forms extremely tight complexes with certain ribonucleases. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA.RI...
, but other proteins such as the tropomyosin
Tropomyosin
Tropomyosin is an actin-binding protein that regulates actin mechanics. It is important, among other things, for muscle contraction. Tropomyosin, along with the troponin complex, associate with actin in muscle fibers and regulate muscle contraction by regulating the binding of myosin...
regulator tropomodulin
Tropomodulin
Tropomodulin is a protein which binds and caps the minus end of actin , regulating the length of actin filaments in muscle and non-muscle cells. The protein functions by physically blocking the spontaneous dissociation of GDP bound actin monomers from the minus end of the actin fibre...
and the toll-like receptor
Toll-like receptor
Toll-like receptors are a class of proteins that play a key role in the innate immune system. They are single, membrane-spanning, non-catalytic receptors that recognize structurally conserved molecules derived from microbes...
also share the motif. In fact, the toll-like receptor
Toll-like receptor
Toll-like receptors are a class of proteins that play a key role in the innate immune system. They are single, membrane-spanning, non-catalytic receptors that recognize structurally conserved molecules derived from microbes...
possesses 10 successive LRR motifs which serve to bind antigen.
Although the canonical LRR protein contains approximately one helix for every beta strand, variants that form beta-alpha superhelix folds sometimes have long loops rather than helices linking successive beta strands.

