
Ribonuclease inhibitor
Encyclopedia
Ribonuclease inhibitor is a large (~450 residues, ~49 kDa), acidic (pI ~4.7), leucine-rich repeat
protein
that forms extremely tight complexes with certain ribonuclease
s. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA
.
RI has a surprisingly high cysteine
content (~6.5%, cf. 1.7% in typical proteins) and is sensitive to oxidation. RI is also rich in leucine
(21.5%, compared to 9% in typical proteins) and commensurately lower in other hydrophobic residues, esp. valine
, isoleucine
, methionine
, tyrosine
, and phenylalanine
.
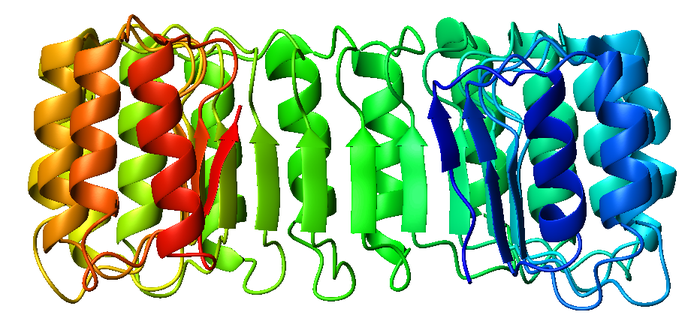 RI is the classic leucine-rich repeat protein, consisting of alternating α-helices
RI is the classic leucine-rich repeat protein, consisting of alternating α-helices
and β-strands
along its backbone. These secondary structure
elements wrap around in a curved, right-handed solenoid that resembles a horseshoe
. The parallel β-strands and α-helices form the inner and outer wall of the horseshoe, respectively. The structure appears to be stabilized by buried asparagine
s at the base of each turn, as it passes from α-helix to β-strand. The αβ repeats alternate between 28 and 29 residues in length, effectively forming a 57-residue unit that corresponds to its genetic structure (each exon
codes for a 57-residue unit).
; the dissociation constant
of the RI-RNase A
complex is roughly 20 fM under physiological conditions while that for the RI-angiogenin complex is even smaller (<1 fM). Remarkably, RI is able to bind a wide variety of RNases, despite having low sequence identity. Structural studies indicate that RNases bind like a "cork in the bottle", associating especially with the C-terminal end of RI; the interaction is largely electrostatic but also buries a lot of surface area (>25 nm2). Efforts to mutate RNases to lower their affinity for RI while maintaining their enzymatic activity have had limited success. However, mammalian RI seems unable to bind a few amphibian ribonucleases, such as ranpirnase
(also known as Onconase).
RI's affinity for ribonucleases is important, since ribonucleases have cytotoxic and cytostatic effects (especially against cancer cells), and are under investigation as potential cancer therapeutics. Successful evasion of the ubiquitous RI would be essential for the success of a ribonuclease drug, (since it would be ineffective bound to RI). The frog protein Onconase is under investigation for treatment of skin cancers; unfortunately, the antigenicity of amphibian proteins makes them unsuitable for treating internal human cancers. Modifications of human ribonucleases that evade RI but retain their enzymatic activity have also been studied.
Leucine-rich repeat
A leucine-rich repeat is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine...
protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
that forms extremely tight complexes with certain ribonuclease
Ribonuclease
Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.-Function:All organisms studied contain...
s. It is a major cellular protein, comprising ~0.1% of all cellular protein by weight, and appears to play an important role in regulating the lifetime of RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
.
RI has a surprisingly high cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
content (~6.5%, cf. 1.7% in typical proteins) and is sensitive to oxidation. RI is also rich in leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
(21.5%, compared to 9% in typical proteins) and commensurately lower in other hydrophobic residues, esp. valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
, isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
, methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, and phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
.
Structure

Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and β-strands
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
along its backbone. These secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
elements wrap around in a curved, right-handed solenoid that resembles a horseshoe
Horseshoe
A horseshoe, is a fabricated product, normally made of metal, although sometimes made partially or wholly of modern synthetic materials, designed to protect a horse's hoof from wear and tear. Shoes are attached on the palmar surface of the hooves, usually nailed through the insensitive hoof wall...
. The parallel β-strands and α-helices form the inner and outer wall of the horseshoe, respectively. The structure appears to be stabilized by buried asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
s at the base of each turn, as it passes from α-helix to β-strand. The αβ repeats alternate between 28 and 29 residues in length, effectively forming a 57-residue unit that corresponds to its genetic structure (each exon
Exon
An exon is a nucleic acid sequence that is represented in the mature form of an RNA molecule either after portions of a precursor RNA have been removed by cis-splicing or when two or more precursor RNA molecules have been ligated by trans-splicing. The mature RNA molecule can be a messenger RNA...
codes for a 57-residue unit).
Binding to ribonucleases
The affinity of RI for ribonucleases is perhaps the highest for any protein-protein interactionProtein-protein interaction
Protein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
; the dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
of the RI-RNase A
Ribonuclease A
Ribonuclease A is a pancreatic ribonuclease that cleaves single-stranded RNA. Bovine pancreatic RNase A is one of the classic model systems of protein science.-History:...
complex is roughly 20 fM under physiological conditions while that for the RI-angiogenin complex is even smaller (<1 fM). Remarkably, RI is able to bind a wide variety of RNases, despite having low sequence identity. Structural studies indicate that RNases bind like a "cork in the bottle", associating especially with the C-terminal end of RI; the interaction is largely electrostatic but also buries a lot of surface area (>25 nm2). Efforts to mutate RNases to lower their affinity for RI while maintaining their enzymatic activity have had limited success. However, mammalian RI seems unable to bind a few amphibian ribonucleases, such as ranpirnase
Ranpirnase
Ranpirnase is a ribonuclease enzyme found in Northern Leopard Frog oocytes. It is being studied in the treatment of cancer, specifically in pleural and peritoneal mesothelioma. It is manufactured by Tamir Biotechnology, Inc. of Monmouth Junction, NJ and known by the ONCONASE trademark...
(also known as Onconase).
RI's affinity for ribonucleases is important, since ribonucleases have cytotoxic and cytostatic effects (especially against cancer cells), and are under investigation as potential cancer therapeutics. Successful evasion of the ubiquitous RI would be essential for the success of a ribonuclease drug, (since it would be ineffective bound to RI). The frog protein Onconase is under investigation for treatment of skin cancers; unfortunately, the antigenicity of amphibian proteins makes them unsuitable for treating internal human cancers. Modifications of human ribonucleases that evade RI but retain their enzymatic activity have also been studied.

