
Liebeskind-Srogl coupling
Encyclopedia
The Liebeskind–Srogl coupling reaction is an organic reaction
forming a new carbon-carbon bond
from a thioester
and a boronic acid
using a metal catalyst. This reaction was invented and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. The metal catalyst depicted below uses TFP = tris(2-furyl)phosphine as an additional ligand and CuTC
= copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.
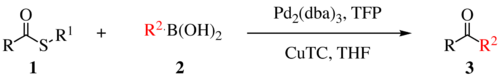
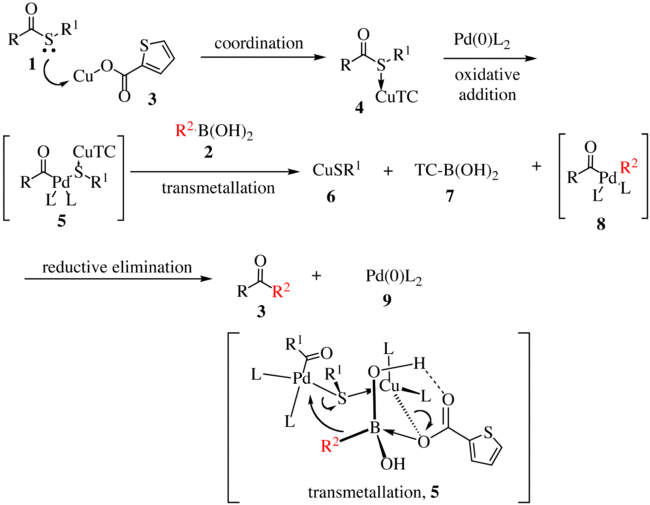
is shown below. The thioester 1 complexes with copper complex 3 to form compound 4. With the oxidative insertion of [Pd] into the carbon-sulfur bond, compound 5 is formed, and with transmetallation, organopalladium
species 8 is formed. The transmetallation proceeds via the transfer of R2 to the palladium metal center with concomitant transfer of the sulfur atom to the copper complex. Reductive elimination gives ketone 3 with the regeneration of the active catalyst 9.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
forming a new carbon-carbon bond
Carbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
from a thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
and a boronic acid
Boronic acid
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
using a metal catalyst. This reaction was invented and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory University, Atlanta, Georgia, USA. The metal catalyst depicted below uses TFP = tris(2-furyl)phosphine as an additional ligand and CuTC
Copper(I)-thiophene-2-carboxylate
Copper-thiophene-2-carboxylate or CuTC is a thiophene and a reagent in organic chemistry that especially promotes the Ullmann reaction between aryl halides....
= copper(I) thiophene-2-carboxylate as a co-metal catalyst. The overall reaction scheme is shown below.
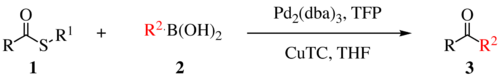
Mechanism
The proposed reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is shown below. The thioester 1 complexes with copper complex 3 to form compound 4. With the oxidative insertion of [Pd] into the carbon-sulfur bond, compound 5 is formed, and with transmetallation, organopalladium
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
species 8 is formed. The transmetallation proceeds via the transfer of R2 to the palladium metal center with concomitant transfer of the sulfur atom to the copper complex. Reductive elimination gives ketone 3 with the regeneration of the active catalyst 9.


