
Limiting oxygen concentration
Encyclopedia
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
below which combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
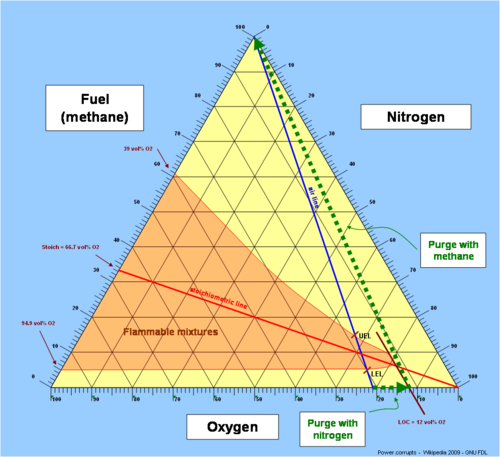
is not possible, independent of the concentration of fuel. It is expressed in units of volume percent of oxygen. The LOC varies with pressure and temperature. It is also dependent on the type of inert (non-flammable) gas.
| Gas or vapor | Nitrogen / Air | Carbon dioxide / Air |
| Hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... | 5 | 5.2 |
||
| Methane Methane Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel... | 12 | 14.5 |
||
| Ethane Ethane Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas.... | 11 | 13.5 |
||
| Propane Propane Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central... | 11.5 | 14.5 |
||
| n-Butane | 12 | 14.5 |
||
| Isobutane Isobutane Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,... | 12 | 15 |
The effect of increasing the concentration of inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
can be understood by viewing the inert as thermal ballast that quenches the flame temperature to a level below which the flame cannot exist. Carbon dioxide is therefore more effective than nitrogen due to its higher molar heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
The concept has important practical use in fire safety engineering. For instance, to safely fill a new container or a pressure vessel with flammable gasses, the atmosphere of normal air (containing about 21 volume percent of oxygen) in the vessel would first be flushed (purged) with nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or another non-flammable inert gas, thereby reducing the oxygen concentration inside the container. When the oxygen concentration is below the LOC, flammable gas can be safely admitted to the vessel because the possibility of internal explosion has been eliminated.
Sources
Monographs Chapter 23(Presented at 9th International Symposium on Loss Prevention and Safety Promotion in the Process Industries, May 1998, Barcelona (Spain))
References'

