
Lincomycin
Encyclopedia
Lincomycin is a lincosamide antibiotic
that comes from the actinomyces Streptomyces lincolnensis
. It has been structurally modified by thionyl chloride
to its more commonly known 7-chloro-7-deoxy derivative, clindamycin
. It is available with the brand name of Lincobect and Lincocin in Pakistan market.
to macrolides, they are also effective against other species as well, i.e., actinomycetes, mycoplasma
, and some species of Plasmodium
.
However, because of its adverse effects and toxicity, it is rarely used today and reserved for patients allergic to penicillin
or where bacteria has developed resistance
.
A two-hour intravenous infusion of 600 mg of Lincomycin achieves average peak serum levels of 15.9 micrograms/ml and yields therapeutic levels for 14 hours for most susceptible gram-positive organisms. Urinary excretion ranges from 4.9 to 30.3 percent (mean: 13.8 percent).
The biological half-life after IM or IV administration is 5.4 ± 1.0 hours. The serum half-life of lincomycin may be prolonged in patients with severe impairment of renal function, compared to patients with normal renal function. In patients with abnormal hepatic function, serum half-life may be twofold longer than in patients with normal hepatic function. Hemodialysis and peritoneal dialysis are not effective in removing lincomycin from the serum.
Tissue level studies indicate that bile is an important route of excretion. Significant levels have been demonstrated in the majority of body tissues. Although lincomycin appears to diffuse in the cerebrospinal fluid (CSF), levels of lincomycin in the CSF appear inadequate for the treatment of meningitis.
classified as a constituent of the lincosamide group, which typically feature a 6,8-dideoxy-6-aminooctose lincosamine. In Lincomycin A, this sugar
moiety (referred to as methylthiolincosamide) is linked via an amide bond to an amino acid derivative (referred to as propylhygric acid). Lincomycin biosynthesis
occurs via a biphasic pathway producing propylproline and methylthiolincosamide followed by condensation of these subunits to N-demethyllincomycin and methyllation by S-adenosylmethionine to produce the antibiotic lincomycin.
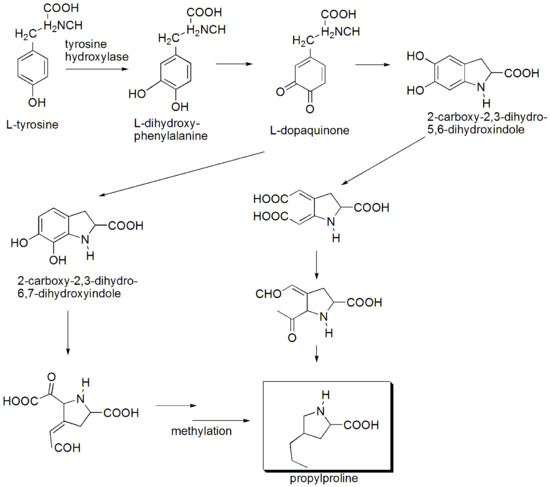
In the biosynthesis
of the amino acid
moiety of lincomycin, tyrosine
comprises seven of the nine carbons in the prophylhygric acid, while the remaining two carbons are added in reactions with S-adenosylmethionine. Glucose
is converted via glycolysis
and the hexose monophosphate pathway to phosphoenolpyruvate and erythrose-4-phosphate, respectively, which are converted via the shikimate pathway to tyrosine and dihydroxyphenylalanine. Although the multistep conversion of dihydroxyphenylalanine to propylproline remains unknown, experiments involving accumulation of 1,2,3,6-tetradehydro-propylproline in mutants lacking a reductase requiring lincomycin cosynthetic factor suggests a biosynthetic scheme that Kuo and coworkers have modified from Brahme et al. to accommodate the remaining steps leading to propylproline.
 The biosynthesis
The biosynthesis
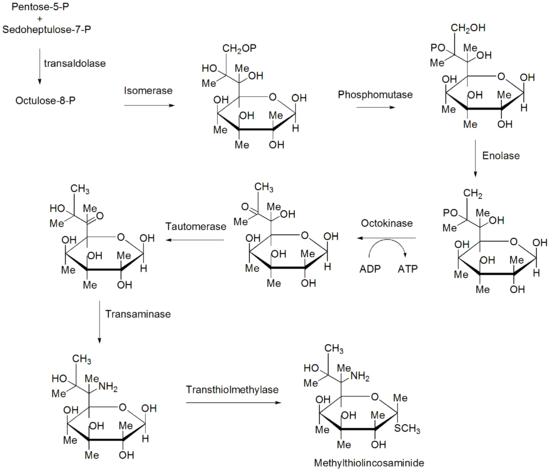
of the methythiolincosamide sugar
moiety is still not entirely known, although two different pathways have been predicted. One possible pathway proposes the C8 carbon framework of methythiolincosamide originates from the condensation of a pentose (C5) unit, stemming from either the hexose monophosphate or condensation through a transketolase reaction with glyceraldehyde-3-phosphate and sedoheptulose-7-phosphate, and a C3 unit, added through a transaldolase reaction with sedoheptulose-7-phosphate. Once condensed, an octose (C8) unit is formed that can undergo isomerization to octose, dephosphorylation and reduction of the C8 carbon, transamination of 6-ketooctose, and thiomethylation of C1 to finally convert the octose unit to the methylthiolincosamide. A substantially different pathway for the formation of the methythiolincosamide proposes that its biosynthesis involves nucleotide activation followed by a series of modifications of dNTP-activated sugar intermediates. Eight genes, lmb-LMNZPOSQ, have been found to form a "sugar subcluster" which might be involved in this sugar metabolism.
 Condensation of both the carboxyl group on the propylproline and the amine group of the methylthiolincosamide via an amide bond is catalyzed by N-Demethyllincomycin-synthetase and leads to the production of N-demethyllincomycin. N-Demethyllincomycin is then methylated by S-adenosylmethionine through N-Demethyllincomycin methyl transferase to form the final lincomycin product.
Condensation of both the carboxyl group on the propylproline and the amine group of the methylthiolincosamide via an amide bond is catalyzed by N-Demethyllincomycin-synthetase and leads to the production of N-demethyllincomycin. N-Demethyllincomycin is then methylated by S-adenosylmethionine through N-Demethyllincomycin methyl transferase to form the final lincomycin product.
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
that comes from the actinomyces Streptomyces lincolnensis
Streptomyces
Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinobacteria, streptomycetes are gram-positive, and have genomes with high guanine and cytosine content...
. It has been structurally modified by thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
to its more commonly known 7-chloro-7-deoxy derivative, clindamycin
Clindamycin
Clindamycin rINN is a lincosamide antibiotic. It is usually used to treat infections with anaerobic bacteria but can also be used to treat some protozoal diseases, such as malaria...
. It is available with the brand name of Lincobect and Lincocin in Pakistan market.
Uses
Although similar in structure, antibacterial spectrum, and in mechanism of actionMechanism of action
In pharmacology, the term mechanism of action refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect...
to macrolides, they are also effective against other species as well, i.e., actinomycetes, mycoplasma
Mycoplasma
Mycoplasma refers to a genus of bacteria that lack a cell wall. Without a cell wall, they are unaffected by many common antibiotics such as penicillin or other beta-lactam antibiotics that target cell wall synthesis. They can be parasitic or saprotrophic. Several species are pathogenic in humans,...
, and some species of Plasmodium
Plasmodium
Plasmodium is a genus of parasitic protists. Infection by these organisms is known as malaria. The genus Plasmodium was described in 1885 by Ettore Marchiafava and Angelo Celli. Currently over 200 species of this genus are recognized and new species continue to be described.Of the over 200 known...
.
However, because of its adverse effects and toxicity, it is rarely used today and reserved for patients allergic to penicillin
Penicillin
Penicillin is a group of antibiotics derived from Penicillium fungi. They include penicillin G, procaine penicillin, benzathine penicillin, and penicillin V....
or where bacteria has developed resistance
Antibiotic resistance
Antibiotic resistance is a type of drug resistance where a microorganism is able to survive exposure to an antibiotic. While a spontaneous or induced genetic mutation in bacteria may confer resistance to antimicrobial drugs, genes that confer resistance can be transferred between bacteria in a...
.
Clinical pharmacology
Intramuscular administration of a single dose of 600 mg of Lincomycin produces average peak serum levels of 11.6 micrograms/ml at 60 minutes, and maintains therapeutic levels for 17 to 20 hours, for most susceptible gram-positive organisms. Urinary excretion after this dose ranges from 1.8 to 24.8 percent (mean: 17.3 percent).A two-hour intravenous infusion of 600 mg of Lincomycin achieves average peak serum levels of 15.9 micrograms/ml and yields therapeutic levels for 14 hours for most susceptible gram-positive organisms. Urinary excretion ranges from 4.9 to 30.3 percent (mean: 13.8 percent).
The biological half-life after IM or IV administration is 5.4 ± 1.0 hours. The serum half-life of lincomycin may be prolonged in patients with severe impairment of renal function, compared to patients with normal renal function. In patients with abnormal hepatic function, serum half-life may be twofold longer than in patients with normal hepatic function. Hemodialysis and peritoneal dialysis are not effective in removing lincomycin from the serum.
Tissue level studies indicate that bile is an important route of excretion. Significant levels have been demonstrated in the majority of body tissues. Although lincomycin appears to diffuse in the cerebrospinal fluid (CSF), levels of lincomycin in the CSF appear inadequate for the treatment of meningitis.
Biosynthesis
Lincomycin is an antibioticAntibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
classified as a constituent of the lincosamide group, which typically feature a 6,8-dideoxy-6-aminooctose lincosamine. In Lincomycin A, this sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
moiety (referred to as methylthiolincosamide) is linked via an amide bond to an amino acid derivative (referred to as propylhygric acid). Lincomycin biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
occurs via a biphasic pathway producing propylproline and methylthiolincosamide followed by condensation of these subunits to N-demethyllincomycin and methyllation by S-adenosylmethionine to produce the antibiotic lincomycin.
In the biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
moiety of lincomycin, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
comprises seven of the nine carbons in the prophylhygric acid, while the remaining two carbons are added in reactions with S-adenosylmethionine. Glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
is converted via glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
and the hexose monophosphate pathway to phosphoenolpyruvate and erythrose-4-phosphate, respectively, which are converted via the shikimate pathway to tyrosine and dihydroxyphenylalanine. Although the multistep conversion of dihydroxyphenylalanine to propylproline remains unknown, experiments involving accumulation of 1,2,3,6-tetradehydro-propylproline in mutants lacking a reductase requiring lincomycin cosynthetic factor suggests a biosynthetic scheme that Kuo and coworkers have modified from Brahme et al. to accommodate the remaining steps leading to propylproline.

Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of the methythiolincosamide sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
moiety is still not entirely known, although two different pathways have been predicted. One possible pathway proposes the C8 carbon framework of methythiolincosamide originates from the condensation of a pentose (C5) unit, stemming from either the hexose monophosphate or condensation through a transketolase reaction with glyceraldehyde-3-phosphate and sedoheptulose-7-phosphate, and a C3 unit, added through a transaldolase reaction with sedoheptulose-7-phosphate. Once condensed, an octose (C8) unit is formed that can undergo isomerization to octose, dephosphorylation and reduction of the C8 carbon, transamination of 6-ketooctose, and thiomethylation of C1 to finally convert the octose unit to the methylthiolincosamide. A substantially different pathway for the formation of the methythiolincosamide proposes that its biosynthesis involves nucleotide activation followed by a series of modifications of dNTP-activated sugar intermediates. Eight genes, lmb-LMNZPOSQ, have been found to form a "sugar subcluster" which might be involved in this sugar metabolism.


