Malic acid
Encyclopedia
Malic acid is an organic compound
with the formula HO2CCH2CHOHCO2H. It is a dicarboxylic acid
which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and ester
s of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle
.
Malate plays an important role in biochemistry
. In the C4 carbon fixation
process, malate is a source of CO2
in the Calvin cycle
. In the citric acid cycle
, (S)-malate is an intermediate, formed by the addition of an -OH
group on the si face of fumarate. It can also be formed from pyruvate via anaplerotic reactions
.
Malate is also synthesized by the carboxylation
of phosphoenolpyruvate
in the guard cells of plant leaves. Malate, as a double anion, often accompanies potassium cations during the uptake of solutes into the guard cells in order to maintain electrical balance in the cell. The accumulation of these solutes within the guard cell decreases the solute potential, allowing water to enter the cell and promote aperture of the stomata.
by Carl Wilhelm Scheele
in 1785. Antoine Lavoisier
in 1787 proposed the name acide malique which is derived from the Latin
word for apple, mālum.
Malic acid contributes to the sourness of green apple
s. It is present in grape
s and in most wines with concentrations sometimes as high as 5 g/l. It confers a tart taste to wine
, although the amount decreases with increasing fruit ripeness. The process of malolactic fermentation
converts malic acid to much milder lactic acid
.
Malic acid, when added to food products, is denoted by E number
E296. Malic acid is the source of extreme tartness in USA-produced confectionery, the so-called extreme candy. It is also used with or in place of the less sour citric acid
in sour sweets. These sweets are sometimes labeled with a warning stating that excessive consumption can cause irritation of the mouth. It is approved for use as a food additive
in the EU, USA and Australia and New Zealand (where it is listed by its INS number 296).
.
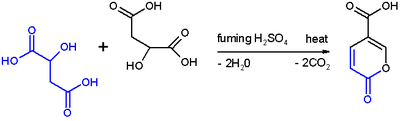
Self-condensation
of malic acid with fuming sulfuric acid gives the pyrone
coumalic acid:
Malic acid was important in the discovery of the Walden inversion
and the Walden cycle, in which (-)-malic acid first is converted into (+)-chlorosuccinic acid by action of phosphorus pentachloride. Wet silver oxide then converts the chlorine compound to (+)-malic acid, which then reacts with PCl5 to the (-)-chlorosuccinic acid. The cycle is completed when silver oxide takes this compound back to (-)-malic acid.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the formula HO2CCH2CHOHCO2H. It is a dicarboxylic acid
Dicarboxylic acid
Dicarboxylic acids are organic compounds that contain two carboxylic acid functional groups. In molecular formulae for dicarboxylic acids, these groups are often written as HOOC-R-COOH, where R may be an alkyl, alkenyl, alkynyl, or aryl group...
which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
.
Biochemistry
L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically.Malate plays an important role in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
. In the C4 carbon fixation
C4 carbon fixation
C4 carbon fixation is one of three biochemical mechanisms, along with and CAM photosynthesis, used in carbon fixation. It is named for the 4-carbon molecule present in the first product of carbon fixation in these plants, in contrast to the 3-carbon molecule products in plants. fixation is an...
process, malate is a source of CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in the Calvin cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
. In the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
, (S)-malate is an intermediate, formed by the addition of an -OH
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group on the si face of fumarate. It can also be formed from pyruvate via anaplerotic reactions
Anaplerotic reactions
Anaplerotic reactions are those that form intermediates of a metabolic pathway. Examples of such are found in the Tricarboxylic acid Cycle...
.
Malate is also synthesized by the carboxylation
Carboxylation
Carboxylation in chemistry is a chemical reaction in which a carboxylic acid group is introduced in a substrate. The opposite reaction is decarboxylation.-Carboxylation in organic chemistry:In organic chemistry many different protocols exist for carboxylation...
of phosphoenolpyruvate
Phosphoenolpyruvate
Phosphoenolpyruvic acid , or phosphoenolpyruvate as the anion, is an important chemical compound in biochemistry. It has the high-energy phosphate bond found in living organisms, and is involved in glycolysis and gluconeogenesis...
in the guard cells of plant leaves. Malate, as a double anion, often accompanies potassium cations during the uptake of solutes into the guard cells in order to maintain electrical balance in the cell. The accumulation of these solutes within the guard cell decreases the solute potential, allowing water to enter the cell and promote aperture of the stomata.
Malic acid in food
Malic acid was first isolated from apple juiceApple juice
Apple juice is a fruit juice manufactured by the maceration and pressing of apples. The resulting expelled juice may be further treated by enzymatic and centrifugal clarification to remove the starch and pectin, which holds fine particulate in suspension, and then pasteurized for packaging in...
by Carl Wilhelm Scheele
Carl Wilhelm Scheele
Carl Wilhelm Scheele was a German-Swedish pharmaceutical chemist. Isaac Asimov called him "hard-luck Scheele" because he made a number of chemical discoveries before others who are generally given the credit...
in 1785. Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
in 1787 proposed the name acide malique which is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
word for apple, mālum.
Malic acid contributes to the sourness of green apple
Apple
The apple is the pomaceous fruit of the apple tree, species Malus domestica in the rose family . It is one of the most widely cultivated tree fruits, and the most widely known of the many members of genus Malus that are used by humans. Apple grow on small, deciduous trees that blossom in the spring...
s. It is present in grape
Grape
A grape is a non-climacteric fruit, specifically a berry, that grows on the perennial and deciduous woody vines of the genus Vitis. Grapes can be eaten raw or they can be used for making jam, juice, jelly, vinegar, wine, grape seed extracts, raisins, molasses and grape seed oil. Grapes are also...
s and in most wines with concentrations sometimes as high as 5 g/l. It confers a tart taste to wine
Wine
Wine is an alcoholic beverage, made of fermented fruit juice, usually from grapes. The natural chemical balance of grapes lets them ferment without the addition of sugars, acids, enzymes, or other nutrients. Grape wine is produced by fermenting crushed grapes using various types of yeast. Yeast...
, although the amount decreases with increasing fruit ripeness. The process of malolactic fermentation
Malolactic fermentation
Malolactic fermentation is a process in winemaking where tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation tends to create a rounder, fuller mouthfeel. It has been said that malic acid tastes of green apples...
converts malic acid to much milder lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
.
Malic acid, when added to food products, is denoted by E number
E number
E numbers are number codes for food additives that have been assessed for use within the European Union . They are commonly found on food labels throughout the European Union. Safety assessment and approval are the responsibility of the European Food Safety Authority...
E296. Malic acid is the source of extreme tartness in USA-produced confectionery, the so-called extreme candy. It is also used with or in place of the less sour citric acid
Citric acid
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks...
in sour sweets. These sweets are sometimes labeled with a warning stating that excessive consumption can cause irritation of the mouth. It is approved for use as a food additive
Food additive
Food additives are substances added to food to preserve flavor or enhance its taste and appearance.Some additives have been used for centuries; for example, preserving food by pickling , salting, as with bacon, preserving sweets or using sulfur dioxide as in some wines...
in the EU, USA and Australia and New Zealand (where it is listed by its INS number 296).
Production and main reactions
Malic acid is produced industrially by the double hydration of maleic anhydrideMaleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
.
Self-condensation
Self-condensation
Self-condensation is an organic reaction in which a chemical compound containing a carbonyl group acts both as the electrophile and the nucleophile in an aldol condensation...
of malic acid with fuming sulfuric acid gives the pyrone
Pyrone
Pyrones or pyranones are a class of cyclic chemical compounds. They contain an unsaturated six membered ring containing one oxygen atom and a ketone functional group. There are two isomers denoted as 2-pyrone and 4-pyrone. The 2-pyrone structure is found in nature as part of the coumarin ring...
coumalic acid:
Malic acid was important in the discovery of the Walden inversion
Walden inversion
Walden inversion is the inversion of a chiral center in a molecule in a chemical reaction. Since a molecule can form two enantiomers around a chiral center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in a SN2 reaction,...
and the Walden cycle, in which (-)-malic acid first is converted into (+)-chlorosuccinic acid by action of phosphorus pentachloride. Wet silver oxide then converts the chlorine compound to (+)-malic acid, which then reacts with PCl5 to the (-)-chlorosuccinic acid. The cycle is completed when silver oxide takes this compound back to (-)-malic acid.