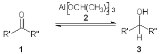
Meerwein-Ponndorf-Verley reduction
Encyclopedia
The Meerwein-Ponndorf-Verley (MPV) Reduction in organic chemistry
is the reduction of ketone
s and aldehyde
s to their corresponding alcohol
s utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol. The beauty in the MPV reduction lies in its high chemoselectivity, and its use of a cheap environmentally friendly metal catalyst.
Figure 1, Exchange of carbonyl oxidation states in the presence of aluminum isopropoxid.
The MPV reduction was discovered by Meerwein
and Schmidt, and separately by Verley in 1925. They found that a mixture of aluminum ethoxide and ethanol could reduce aldehydes to their alcohols. Ponndorf applied the reaction to ketones and upgraded the catalyst to aluminum isopropoxide in isopropanol.
. At this point the new carbonyl dissociates and gives the tricoordinated aluminum species 4. Finally, an alcohol from solution displaces the newly reduced carbonyl to regenerate the catalyst 1.
Figure 2, Catalytic Cycle of Meerwein-Ponndorf-Verley Reduction
Each step in the cycle is reversible and the reaction is driven by the thermodynamic properties of the intermediates and the products. This means that given time the more thermodynamically stable product will be favored.
Several other mechanisms have been proposed for this reaction, including a radical
mechanism as well as a mechanism involving an aluminum hydride species. The direct hydride
transfer is the commonly accepted mechanism recently supported by experimental and theoretical data.
. Aldehydes are reduced before ketones allowing for a measure of control over the reaction. If it is necessary to reduce one carbonyl in the presence of another, the common carbonyl protecting groups may be employed. Groups, such as alkenes and alkynes, that normally pose a problem for reduction by other means have no reactivity under these conditions.
can be performed on prochiral ketones leading to chiral alcohols. The three main ways to achieve the asymmetric reduction is by use of a chiral alcohol hydride source, use of an intramolecular MPV reduction, or use of a chiral ligand on the aluminum alkoxide.
One method of achieving the asymmetric MPV reduction is with the use of chiral hydride donating alcohols. The use of chiral alcohol (R)-(+)-sec-o-bromophen-ethyl alcohol gave 82%ee (percent enantiomeric excess
) in the reduction of 2-chloroacetophenone . This enantioselection is due to the sterics of the two phenol groups in the six membered transition state as shown in Figure 3. In Figure 3, 1 is favored over 2 due to the large steric effect in 2 from the two phenyl groups.
Figure 3, Transition states of MPV reduction with a chiral alcohol
The use of an intramolecular MPV reduction can give good enantiopurity . By tethering the ketone to the hydride source only one transition state is possible (Figure 4) leading to the asymmetric reduction. This method, however, has the ability to undergo the reverse Oppenauer Oxidation
due to the proximity of the two reagents. Thus the reaction runs under thermodynamic equilibrium with the ratio of the products related to their relative stabilities. After the reaction is run the hydride-source potrion of the molecule can be removed.
Figure4, Transition state of intramolecular MPV reduction
Chiral ligand
s on the aluminum alkoxide can effect the stereochemical outcome of the MPV reduction. This method lead to the reduction of substituted acetophenone
s in up to 83%ee (Figure 5). The appeal of this method is that it uses a chiral ligand as opposed to a stoiciometric
source of chirality. It has been recently shown that the low selectivity of this method is due to the shape of the transition state. It has been shown that the transition state is a planer six member transition state. This is different than the believed Zimmerman-Traxler model like transition state.
Figure 5, MPV reaction with chiral ligand
While commercial aluminim isopropoxide is available, the use of it often requires catalyst loadings of up to 100-200 mol%. This hinders the use of the MPV reduction on scale. Recent work has shown that aluminum alkoxides made in situ from trimethyl aluminum reagents have far better activity requiring as little as 10% loading. The activity difference is believed to be due to the large aggregation state of the commercially available product.
Several side reactions are known to occur. In the case of ketones and especially aldehydes aldol condensation
s have been observed. Aldehydes with no α-hydrogens can undergo the Tishchenko reaction
. Finally, in some cases the alcohol generated by the reduction can be dehydrated giving an alkyl carbon.
s from ketimines using a chiral alkoxide. The addition of a phosphinoyl group to the nitrogen of the ketimine allowed for high enantioselectivity up to 98%ee .
Work has been done in the use of lanthanide
s for the Meerwein-Ponndorf-Verley Reduction. Both Ruthenium
and Samarium
have shown high yields and high stereoselectivity in the reduction of carbonyls to alcohols. The Ruthenium catalyst has been shown, however, to go through a Ruthenium hydride intermediate.
The standard MPV reduction is a homogeneous reaction several heterogeneous reactions have been developed.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is the reduction of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s and aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s to their corresponding alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol. The beauty in the MPV reduction lies in its high chemoselectivity, and its use of a cheap environmentally friendly metal catalyst.
The MPV reduction was discovered by Meerwein
Hans Meerwein
Hans Meerwein was a German chemist.His name is present in the names of several reactions and reagents, for example the Meerwein-Ponndorf-Verley reduction, the Wagner-Meerwein rearrangement...
and Schmidt, and separately by Verley in 1925. They found that a mixture of aluminum ethoxide and ethanol could reduce aldehydes to their alcohols. Ponndorf applied the reaction to ketones and upgraded the catalyst to aluminum isopropoxide in isopropanol.
Mechanism
The MPV reduction is believed to go through a catalytic cycle involving a six member ring transition state as shown in Figure 2. Starting with the aluminum alkoxide 1, a carbonyl oxygen is coordinated to achieve the tetra coordinated aluminum intermediate 2. Between intermediates 2 and 3 the hydride is transferred to the carbonyl from the alkoxy ligand via a pericyclic mechanismPericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
. At this point the new carbonyl dissociates and gives the tricoordinated aluminum species 4. Finally, an alcohol from solution displaces the newly reduced carbonyl to regenerate the catalyst 1.
Each step in the cycle is reversible and the reaction is driven by the thermodynamic properties of the intermediates and the products. This means that given time the more thermodynamically stable product will be favored.
Several other mechanisms have been proposed for this reaction, including a radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
mechanism as well as a mechanism involving an aluminum hydride species. The direct hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
transfer is the commonly accepted mechanism recently supported by experimental and theoretical data.
Chemoselectivity
One of the great draws of the Meewein-Ponndorf-Verley reduction is its chemoselectivityChemoselectivity
Chemical reactions are defined usually in small contexts , such generalizations are a matter of utility. The preferential outcome of one instance of a generalized reaction over a set of other plausible reactions, is defined as chemoselectivity...
. Aldehydes are reduced before ketones allowing for a measure of control over the reaction. If it is necessary to reduce one carbonyl in the presence of another, the common carbonyl protecting groups may be employed. Groups, such as alkenes and alkynes, that normally pose a problem for reduction by other means have no reactivity under these conditions.
Stereoselectivity
The aluminum based Meerwein-Ponndorf-Verley reductionMeerwein-Ponndorf-Verley reduction
The Meerwein-Ponndorf-Verley Reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol...
can be performed on prochiral ketones leading to chiral alcohols. The three main ways to achieve the asymmetric reduction is by use of a chiral alcohol hydride source, use of an intramolecular MPV reduction, or use of a chiral ligand on the aluminum alkoxide.
One method of achieving the asymmetric MPV reduction is with the use of chiral hydride donating alcohols. The use of chiral alcohol (R)-(+)-sec-o-bromophen-ethyl alcohol gave 82%ee (percent enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
) in the reduction of 2-chloroacetophenone . This enantioselection is due to the sterics of the two phenol groups in the six membered transition state as shown in Figure 3. In Figure 3, 1 is favored over 2 due to the large steric effect in 2 from the two phenyl groups.
The use of an intramolecular MPV reduction can give good enantiopurity . By tethering the ketone to the hydride source only one transition state is possible (Figure 4) leading to the asymmetric reduction. This method, however, has the ability to undergo the reverse Oppenauer Oxidation
Oppenauer oxidation
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...
due to the proximity of the two reagents. Thus the reaction runs under thermodynamic equilibrium with the ratio of the products related to their relative stabilities. After the reaction is run the hydride-source potrion of the molecule can be removed.
Chiral ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s on the aluminum alkoxide can effect the stereochemical outcome of the MPV reduction. This method lead to the reduction of substituted acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
s in up to 83%ee (Figure 5). The appeal of this method is that it uses a chiral ligand as opposed to a stoiciometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
source of chirality. It has been recently shown that the low selectivity of this method is due to the shape of the transition state. It has been shown that the transition state is a planer six member transition state. This is different than the believed Zimmerman-Traxler model like transition state.
Scope
Several problems restrict the use of the Meerwein–Ponndorf–Verley reduction compared to the use of other reducing agents. The stereochemical control is seriously limited. Oftentimes a large amount of aluminimum alkoxide is needed when using commercial reagent, and there are several known side reactions.While commercial aluminim isopropoxide is available, the use of it often requires catalyst loadings of up to 100-200 mol%. This hinders the use of the MPV reduction on scale. Recent work has shown that aluminum alkoxides made in situ from trimethyl aluminum reagents have far better activity requiring as little as 10% loading. The activity difference is believed to be due to the large aggregation state of the commercially available product.
Several side reactions are known to occur. In the case of ketones and especially aldehydes aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
s have been observed. Aldehydes with no α-hydrogens can undergo the Tishchenko reaction
Tishchenko reaction
The Tishchenko reaction is a chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide. The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides...
. Finally, in some cases the alcohol generated by the reduction can be dehydrated giving an alkyl carbon.
Variations
The Meerwein–Ponndorf–Verley reduction has been recently used in the synthesis of chiral amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s from ketimines using a chiral alkoxide. The addition of a phosphinoyl group to the nitrogen of the ketimine allowed for high enantioselectivity up to 98%ee .
Work has been done in the use of lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s for the Meerwein-Ponndorf-Verley Reduction. Both Ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
and Samarium
Samarium
Samarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3...
have shown high yields and high stereoselectivity in the reduction of carbonyls to alcohols. The Ruthenium catalyst has been shown, however, to go through a Ruthenium hydride intermediate.
The standard MPV reduction is a homogeneous reaction several heterogeneous reactions have been developed.

