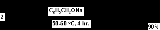
Tishchenko reaction
Encyclopedia
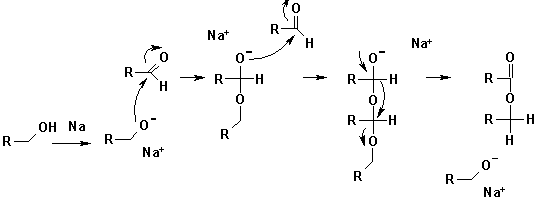
The Tishchenko reaction is a chemical reaction
that involves disproportionation
of an aldehyde
lacking a hydrogen atom in the alpha position in the presence of an alkoxide
. The reaction product is an ester
. Catalysts are aluminium alkoxides or sodium alkoxides. Benzaldehyde
reacts with sodium benzyloxide (generated from sodium
and benzyl alcohol
) to benzyl benzoate
.
 Paraformaldehyde
Paraformaldehyde
reacts with boric acid
to methyl formate
. The key step in the reaction mechanism
for this reaction is a 1,3-hydride shift in the hemiacetal
intermediate formed from two successive nucleophilic addition
reactions, the first one from the catalyst. The hydride shift regenerates the alkoxide catalyst.
 In the related Cannizzaro reaction
In the related Cannizzaro reaction
the base is sodium hydroxide and then the oxidation product is a carboxylic acid
and the reduction product is an alcohol
.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that involves disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
lacking a hydrogen atom in the alpha position in the presence of an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
. The reaction product is an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
. Catalysts are aluminium alkoxides or sodium alkoxides. Benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
reacts with sodium benzyloxide (generated from sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and benzyl alcohol
Benzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
) to benzyl benzoate
Benzyl benzoate
Benzyl benzoate is the ester of benzyl alcohol and benzoic acid, with the formula C6H5CH2O2CC6H5. This easily prepared compound has a variety of uses.-Synthesis:This colorless liquid is formally the condensation product of benzoic acid and benzyl alcohol...
.

Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
reacts with boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
to methyl formate
Methyl formate
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a clear liquid with an ethereal odor, high vapor pressure, and low surface tension.-Production:...
. The key step in the reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for this reaction is a 1,3-hydride shift in the hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
intermediate formed from two successive nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
reactions, the first one from the catalyst. The hydride shift regenerates the alkoxide catalyst.

Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
the base is sodium hydroxide and then the oxidation product is a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
and the reduction product is an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
.
Related reactions
- Aldol-Tishchenko reactionAldol-Tishchenko reactionThe Aldol-Tishchenko reaction is a tandem reaction involving an Aldol reaction and a Tishchenko reaction. In organic synthesis it is a method to convert aldehydes and ketones into 1,3-hydroxyl compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by...
- Baylis-Hillman reaction
- Cannizzaro reactionCannizzaro reactionThe Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
- Meerwein-Ponndorf-Verley ReductionMeerwein-Ponndorf-Verley reductionThe Meerwein-Ponndorf-Verley Reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol...
- Oppenauer OxidationOppenauer oxidationOppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...

