
Monosaccharide nomenclature
Encyclopedia
Monosaccharide nomenclature is a set of conventions used in chemistry
to name the compounds
known as monosaccharides or "simple sugars" — the basic structural units of carbohydrate
s, which cannot be hydrolysed
into simpler units.
atoms n: trioses, tetroses, pentoses, hexoses, etc.
Every simple monosaccharide has an acyclic
(open chain) form, which can be written as H-(CH(OH))x-(C=O)-(CH(OH))y-H; that is, a straight chain of carbon atoms, one of which is a carbonyl
group
, all the others bearing an hydrogen -H and a hydroxyl
-OH each, with one extra hydrogen at either end. The carbons of the chain are conventionally numbered from 1 to n, starting from the end which is closest to the carbonyl.
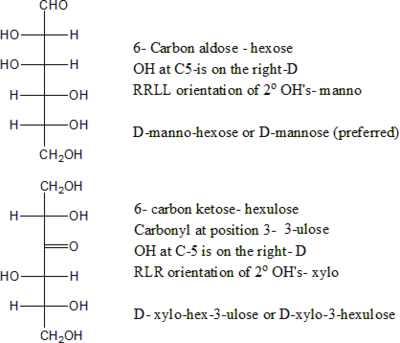
If the carbonyl is at the very beginning of the chain (carbon 1), the monosaccharide is said to be an aldose
, otherwise it is a ketose
. These names can be combined with the chain length prefix, as in aldohexose
or ketopentose. Most ketoses found in nature have the carbonyl in position 2; when that is not the case, one uses a numeric prefix to indicate the carbonyl's position. Thus for example, aldohexose means H(C=O)(CHOH)5H, ketopentose means H(CHOH)(C=O)(CHOH)3H, and 3-ketopentose means H(CHOH)2(C=O)(CHOH)2H.
An alternative nomenclature uses the suffix '-ose' only for aldoses, and '-ulose' for ketoses. The position of the carbonyl (when it is not 1 or 2) is indicated by a numerical infix. For example, hexose in this nomenclature means H(C=O)(CHOH)5H, pentulose means H(CHOH)(C=O)(CHOH)3H, and hexa-3-ulose means H(CHOH)2(C=O)(CHOH)3H.
is a systematic way of drawing the skeletal formula
of an open-chain monosaccharide so that each stereoisomer is uniquely identified.
Two isomers whose molecules are mirror-images of each other are identified by prefixes 'D-' or 'L-', according to the handedness of the chiral carbon atom that is farthest from the carbonyl. In the Fischer projection, that is the second carbon from the bottom; the prefix is 'D-' or 'L-' according to whether the hydroxyl on that carbon lies to the right or left of the backbone, respectively.
If the molecular graph is symmetrical (H(CHOH)x(CO)(CHOH)xH) and the two halves are mirror images of each other, then the molecule is identical to its mirror image, and there is no 'L-' form.
A distinct common name, such as "glucose" or "ribose", is traditionally assigned to each pair of mirror-image stereoisomers, and to each achiral stereoisomer. These names have standard three-letter abbreviations, such as 'Glu' for glucose and 'Rib' for ribose.
Another nomenclature uses the systematic name of the molecular graph, a 'D-' or 'L-' prefix to indicate the position of the last chiral hydroxyl on the Fischer diagram (as above), and another italic prefix to indicate the positions of the remaining hydroxyls relative to the first one, read from bottom to top in the diagram, skipping the keto group if any. These prefixes are attached to the systematic name of the molecular graph. So for example, D-glucose is D-gluco-hexose, D-ribose is D-ribo-pentose, and D-psicose is D-ribo-hexulose. Note that, in this nomenclature, mirror-image isomers differ only in the 'D'/'L' prefix, even though all their hydroxyls are reversed.
 The following tables shows the Fischer projections of selected monosaccharides (in open-chain form), with their conventional names. The table shows all aldoses with 3 to 6 carbon atoms, and a few ketoses. For chiral molecules, only the 'D-' form (with the next-to-last hydroxyl on the right side) is shown; the corresponding forms have mirror-image structures. Some of these monosaccharides are only synthetically prepared in the laboratory and not found in nature.
The following tables shows the Fischer projections of selected monosaccharides (in open-chain form), with their conventional names. The table shows all aldoses with 3 to 6 carbon atoms, and a few ketoses. For chiral molecules, only the 'D-' form (with the next-to-last hydroxyl on the right side) is shown; the corresponding forms have mirror-image structures. Some of these monosaccharides are only synthetically prepared in the laboratory and not found in nature.
s in their cyclic form, an infix is placed before the '-ose', '-ulose', or n-ulose' suffix to specify the ring size. The infix is "furan" for a 5-atom ring, "pyran" for 6, "septan" for 7, and so on).
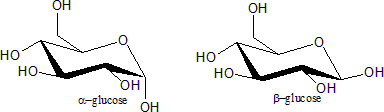
Ring closure creates another chiral center at the anomeric carbon (the one with the hemiacetal
or acetal
functionality), and therefore each open-chain stereoisomer gives rise to two distinct stereoisomers (anomer
s). These are identified by the prefixes 'α-' and 'β-', according to he configuration of the anomeric carbon relative to that of furthest stereocenter along the open chain. To determine if the sugar is α or β, the structure is drawn in a Fischer projection
; if the endocyclic oxygen (O5) and exocyclic oxygen (O1) are cis, the sugar is α; if they are trans, the sugar is β.
 Examples
Examples

HOR, followed by the saccahride name with the '-e' ending repalced by '-ide'; as in phenol D-glucopyrnoside.
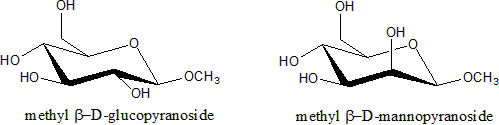
Rules for Nomenclature of Modified Sugars:
Examples
Rules for Nomenclature for Protected Sugars:

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
to name the compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
known as monosaccharides or "simple sugars" — the basic structural units of carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s, which cannot be hydrolysed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
into simpler units.
Systematic name of molecular graph
The elementary formula of a simple monosaccharide is CnH2nOn, where the integer n is at least 3 and rarely greater than 7. Simple monosaccharides may be named generically according on the number of carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms n: trioses, tetroses, pentoses, hexoses, etc.
Every simple monosaccharide has an acyclic
Acyclic
Acyclic can refer to:* In chemistry, a compound which is not cyclic, e.g. alkanes and acyclic aliphatic compounds* In mathematics:** A graph without a cycle, especially*** A directed acyclic graph...
(open chain) form, which can be written as H-(CH(OH))x-(C=O)-(CH(OH))y-H; that is, a straight chain of carbon atoms, one of which is a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, all the others bearing an hydrogen -H and a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
-OH each, with one extra hydrogen at either end. The carbons of the chain are conventionally numbered from 1 to n, starting from the end which is closest to the carbonyl.
If the carbonyl is at the very beginning of the chain (carbon 1), the monosaccharide is said to be an aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
, otherwise it is a ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
. These names can be combined with the chain length prefix, as in aldohexose
Aldohexose
An aldohexose is a hexose with an aldehyde group on one end.The aldohexoses have four chiral centres for a total of 16 possible aldohexose stereoisomers . Of these, only three commonly occur in nature: D-glucose, D-galactose, and D-mannose...
or ketopentose. Most ketoses found in nature have the carbonyl in position 2; when that is not the case, one uses a numeric prefix to indicate the carbonyl's position. Thus for example, aldohexose means H(C=O)(CHOH)5H, ketopentose means H(CHOH)(C=O)(CHOH)3H, and 3-ketopentose means H(CHOH)2(C=O)(CHOH)2H.
An alternative nomenclature uses the suffix '-ose' only for aldoses, and '-ulose' for ketoses. The position of the carbonyl (when it is not 1 or 2) is indicated by a numerical infix. For example, hexose in this nomenclature means H(C=O)(CHOH)5H, pentulose means H(CHOH)(C=O)(CHOH)3H, and hexa-3-ulose means H(CHOH)2(C=O)(CHOH)3H.
Naming of acyclic stereoisomers
Open-chain monosaccharides with same molecular graph may exist as two or more stereoisomers. The Fischer projectionFischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
is a systematic way of drawing the skeletal formula
Skeletal formula
The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and...
of an open-chain monosaccharide so that each stereoisomer is uniquely identified.
Two isomers whose molecules are mirror-images of each other are identified by prefixes 'D-' or 'L-', according to the handedness of the chiral carbon atom that is farthest from the carbonyl. In the Fischer projection, that is the second carbon from the bottom; the prefix is 'D-' or 'L-' according to whether the hydroxyl on that carbon lies to the right or left of the backbone, respectively.
If the molecular graph is symmetrical (H(CHOH)x(CO)(CHOH)xH) and the two halves are mirror images of each other, then the molecule is identical to its mirror image, and there is no 'L-' form.
A distinct common name, such as "glucose" or "ribose", is traditionally assigned to each pair of mirror-image stereoisomers, and to each achiral stereoisomer. These names have standard three-letter abbreviations, such as 'Glu' for glucose and 'Rib' for ribose.
Another nomenclature uses the systematic name of the molecular graph, a 'D-' or 'L-' prefix to indicate the position of the last chiral hydroxyl on the Fischer diagram (as above), and another italic prefix to indicate the positions of the remaining hydroxyls relative to the first one, read from bottom to top in the diagram, skipping the keto group if any. These prefixes are attached to the systematic name of the molecular graph. So for example, D-glucose is D-gluco-hexose, D-ribose is D-ribo-pentose, and D-psicose is D-ribo-hexulose. Note that, in this nomenclature, mirror-image isomers differ only in the 'D'/'L' prefix, even though all their hydroxyls are reversed.

Names of aldoses
| Aldotrioses Trioses |
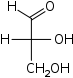 D-Glyceraldehyde Glyceraldehyde Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism... |
|||||||
| Aldotetroses Tetroses |
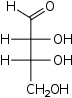 D-Erythrose Erythrose Erythrose is a tetrose carbohydrate with chemical formula C4H8O4. It has one aldehyde group and so is part of the aldose family. The natural isomer is D-erythrose.... erythro- |
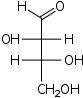 D-Threose Threose Threose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides... threo- |
||||||
| Aldopentoses Pentoses |
 D-Ribose Ribose Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection.... ribo- Rib |
 D-Arabinose Arabinose Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde functional group.For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, or structurally analogous to D-glyceraldehyde.For sugars, the D/L... arabino- Ara |
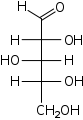 D-Xylose Xylose Xylose is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is the precursor to hemicellulose, one of the main constituents of biomass... xylo- Xyl |
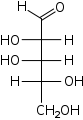 D-Lyxose Lyxose Lyxose is an aldopentose — a monosaccharide containing five carbon atoms, and including an aldehyde functional group. It has chemical formula 5105.Lyxose occurs only rarely in nature, for example, as a component of bacterial glycolipids.- See also :... lyxo- Lyx |
||||
| Aldohexoses Hexoses |
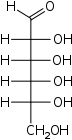 D-Allose Allose Allose is an aldohexose sugar. It is a rare monosaccharide that has been isolated from the leaves of the African shrub Protea rubropilosa. It is soluble in water and practically insoluble in methanol.Allose is a C-3 epimer of glucose.... allo- Ala |
 D-Altrose Altrose Altrose is an aldohexose sugar. D-Altrose is an unnatural monosaccharide. It is soluble in water and practically insoluble in methanol. However, L-altrose has been isolated from strains of the bacterium Butyrivibrio fibrisolvens.... altro- Alt |
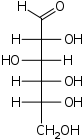 D-Glucose Glucose Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate... gluco- Glc |
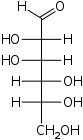 D-Mannose Mannose Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics.... manno- Man |
 D-Gulose Gulose Gulose is an aldohexose sugar. It is a monosaccharide that is very rare in nature, but has been found in archaea, bacteria and eukaryotes. It also exists as a syrup with a sweet taste. It is soluble in water and slightly soluble in methanol. Both the D- and L-forms are not fermentable by... gulo- Gul |
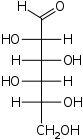 D-Idose Idose Idose is a hexose, a six carbon monosaccharide. It has an aldehyde group and is an aldose. It is not found in nature, but its uronic acid, iduronic acid, is important. It is a component of dermatan sulfate and heparan sulfate, which are glycosaminoglycans. The first and third hydroxyls point the... ido- Ido |
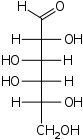 D-Galactose Galactose Galactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose.... galacto- Gal |
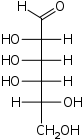 D-Talose Talose Talose is an aldohexose sugar. It is an unnatural monosaccharide that is soluble in water and slightly soluble in methanol. Some etymologists suggest that talose's name derives from the automaton of Greek mythology named Talos, but the relevance is unclear.... talo- Tal |
Names of ketoses
| Ketotrioses Triuloses |
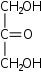 Glycerone Dihydroxyacetone Dihydroxyacetone , or DHA, also known as glycerone, is a simple carbohydrate with formula .DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, and by the fermentation of glycerin.-Chemistry:DHA is a... |
|||
| Ketotetrose Tetruloses |
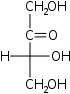 D-Erythrulose Erythrulose D-Erythrulose is a tetrose carbohydrate with the chemical formula C4H8O4. It has one ketone group and so is part of the ketose family... glycero- |
|||
| Ketopentoses Pentuloses |
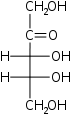 D-Ribulose Ribulose Ribulose is a ketopentose — a monosaccharide containing five carbon atoms, and including a ketone functional group. It has chemical formula 5105. Two enantiomers are possible, D-ribulose and L-ribulose . D-Ribulose is the diastereomer of D-xylulose.Ribulose sugars are composed in the... erythro- Rul |
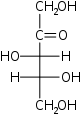 D-Xylulose Xylulose Xylulose is a ketopentose, a monosaccharide containing five carbon atoms, and including a ketone functional group. It has the chemical formula 5105. In nature, it occurs in both the L- and D-enantiomers.- Pathology :... threo- Xul |
||
| Ketohexoses Hexuloses |
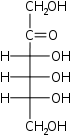 D-Psicose Psicose D-Psicose is an ultralow-energy monosaccharide sugar. It is a C-3 epimer of D-fructose, and is present in small quantities in agricultural products and commercially-prepared carbohydrate complexes. It is known as a "rare sugar" because it is rarely found in nature, and even when found, only in... ribo- Psi |
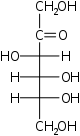 D-Fructose Fructose Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847... arabino- Fru |
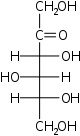 D-Sorbose Sorbose Sorbose is a ketose belonging to the group of sugars known as monosaccharides. It has a sweetness that is equivalent to sucrose . The commercial production of vitamin C often begins with sorbose. L-Sorbose is the configuration of the naturally occurring sugar.... xylo- Sor |
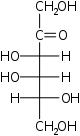 D-Tagatose Tagatose Tagatose is a functional sweetener. It is a naturally occurring monosaccharide, specifically a hexose. It is often found in dairy products, and is very similar in texture to sucrose and is 92% as sweet, but with only 38% of the calories.... lyxo- Tag |
Names of 3-ketoses
| 3-Ketopentoses Penta-3-uloses |
SYM-3-Ketopentose |
D-UNS-3-Ketopentose |
||||||
| 3-Ketohexoses Hexa-3-uloses |
D-RRR-3-Ketohexose |
D-RRL-3-Ketohexose |
D-RLR-3-Ketohexose |
D-RLL-3-Ketohexose |
D-LRR-3-Ketohexose |
D-LRL-3-Ketohexose |
D-LLR-3-Ketohexose |
D-LLL-3-Ketohexose |
Cyclic forms
For monosaccharideMonosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s in their cyclic form, an infix is placed before the '-ose', '-ulose', or n-ulose' suffix to specify the ring size. The infix is "furan" for a 5-atom ring, "pyran" for 6, "septan" for 7, and so on).
Ring closure creates another chiral center at the anomeric carbon (the one with the hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
or acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
functionality), and therefore each open-chain stereoisomer gives rise to two distinct stereoisomers (anomer
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
s). These are identified by the prefixes 'α-' and 'β-', according to he configuration of the anomeric carbon relative to that of furthest stereocenter along the open chain. To determine if the sugar is α or β, the structure is drawn in a Fischer projection
Fischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
; if the endocyclic oxygen (O5) and exocyclic oxygen (O1) are cis, the sugar is α; if they are trans, the sugar is β.


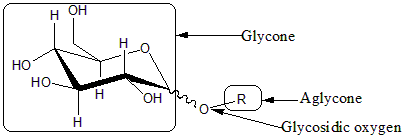
Glycosides
Glycosides are saccharides in which the hydroxyl -OH at the anomeric centre is replaced by an oxygen-bridged group -OR. The carbohydrate part of the molecule is called glycone, the -O- bridge is the glycosisdic oxygen, and the attached group is the aglycone. Glycosides are named by giving the aglyconic alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
HOR, followed by the saccahride name with the '-e' ending repalced by '-ide'; as in phenol D-glucopyrnoside.

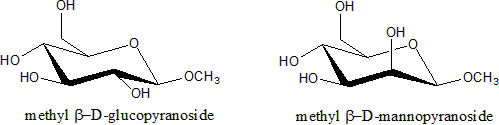
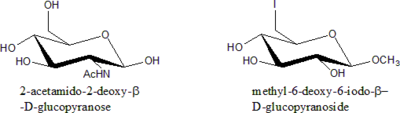
Deoxy sugars
Modification of sugar is generally done by replacing one or more –OH group with other functional groups at all position except C-1. Since all these cases involves the removal of an –OH group, they are all deoxy sugars.Rules for Nomenclature of Modified Sugars:
- State the Sugar is deoxy sugar.
- Specify the position of deoxygenationDeoxygenationDeoxygenation is a chemical reaction involving the removal of molecular oxygen from a reaction mixture or solvent, or the removal of oxygen atoms from a molecule.Classic representatives of deoxygenation are:...
. - If there is a substituent other than H in the place of –OH, specify what it is.
- Specify the relative configuration of all stereogenic centres (manno, gluco etc.).
- Specify the ring size (furanose, pyranose etc.) and anomeric configuration ( a or b).
- State the chain length only in situation where –OH is replaced with H.
- Alphabetize all the substituent groups (deoxy, -iodo, -amino etc.). Di-, tri- etc. prefixes do not count.
Examples

Protected Sugars
Sugars in which –OH is protected by some modification are called protected sugars.Rules for Nomenclature for Protected Sugars:
- Specify the number of particular protecting groups (di, tri, tetra etc.).
- List groups alphabetically along with all other substituents ( di, tri prefixes do not count).

See also
- Carbohydrate conformationCarbohydrate conformationCarbohydrate conformation is the characteristic 3-dimensional shape of a carbohydrate. Conformations of monosaccharide and oligosaccharide heavily influence their reactivity and recognition by other molecules, which are essential to mammals and other organisms....
- PolysaccharidePolysaccharidePolysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
- OligosaccharideOligosaccharideAn oligosaccharide is a saccharide polymer containing a small number of component sugars, also known as simple sugars...
- Oligosaccharide nomenclatureOligosaccharide nomenclatureOligosaccharides and polysaccharides are an important class of polymeric carbohydrates found in virtually all living entities. Their structural features make their nomenclature challenging and their roles in living systems make their nomenclature important.- Oligosaccharides :Oligosaccharides are...

