Mössbauer effect
Encyclopedia
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil
-free emission and absorption of γ radiation
by atomic nuclei bound in a solid. Its main application is in Mössbauer spectroscopy.
s by gases had been observed previously, and it was expected that a similar phenomenon would be found for gamma ray
s, which are created by nuclear
transitions (as opposed to x-rays, which are typically produced by electron
ic transitions). However, attempts to observe gamma-ray resonance in gases failed due to energy being lost to recoil, preventing resonance (the Doppler effect
also broadens the gamma-ray spectrum). Mössbauer was able to observe resonance in solid iridium
, which raised the question of why gamma-ray resonance was possible in solids, but not in gases. Mössbauer proposed that, for the case of atoms bound into a solid, under certain circumstances a fraction of the nuclear events could occur essentially without recoil. He attributed the observed resonance to this recoil-free fraction of nuclear events.
The Mössbauer effect was one of the last major discoveries in physics to be originally reported in German language. The first report in English language was a letter describing a repetition of the experiment.
The discovery was rewarded with the Nobel Prize in Physics
in 1961 together with Robert Hofstadter
's research of electron scattering
in atomic nuclei.
 In general, gamma rays are produced by nuclear transitions from an unstable high-energy state, to a stable low-energy state. The energy of the emitted gamma ray corresponds to the energy of the nuclear transition, minus an amount of energy that is lost as recoil to the emitting atom. If the lost "recoil energy" is small compared with the energy linewidth of the nuclear transition, then the gamma ray energy still corresponds to the energy of the nuclear transition, and the gamma ray can be absorbed by a second atom of the same type as the first. This emission and subsequent absorption is called resonance. Additional recoil energy is also lost during absorption, so in order for resonance to occur the recoil energy must actually be less than half the linewidth for the corresponding nuclear transition.
In general, gamma rays are produced by nuclear transitions from an unstable high-energy state, to a stable low-energy state. The energy of the emitted gamma ray corresponds to the energy of the nuclear transition, minus an amount of energy that is lost as recoil to the emitting atom. If the lost "recoil energy" is small compared with the energy linewidth of the nuclear transition, then the gamma ray energy still corresponds to the energy of the nuclear transition, and the gamma ray can be absorbed by a second atom of the same type as the first. This emission and subsequent absorption is called resonance. Additional recoil energy is also lost during absorption, so in order for resonance to occur the recoil energy must actually be less than half the linewidth for the corresponding nuclear transition.
The amount of energy in the recoiling body can be found from momentum conservation:

where is the momentum of the recoiling matter, and the momentum of the gamma ray. Substituting energy into the equation gives:
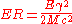
where ( for ) is the energy lost as recoil, is the energy of the gamma ray ( for ), ( for ) is the mass of the emitting or absorbing body, and c is the speed of light
. In the case of a gas the emitting and absorbing bodies are atoms, so the mass is quite small, resulting in a large recoil energy, which prevents resonance. (Note that the same equation applies for recoil energy losses in x-rays, but the photon energy is much less, resulting in a lower energy loss, which is why gas-phase resonance could be observed with x-rays.)
In a solid, the nuclei are bound to the lattice and do not recoil in the same way as in a gas. The lattice as a whole recoils but the recoil energy is negligible because the in the above equation is the mass of the whole lattice. However, the energy in a decay can be taken up or supplied by lattice vibrations. The energy of these vibrations is quantised in units known as phonons. The Mössbauer effect occurs because there is a finite probability of a decay occurring involving no phonons. Thus in a fraction of the nuclear events (the recoil-free fraction, given by the Lamb–Mössbauer factor
), the entire crystal acts as the recoiling body, and these events are essentially recoil-free. In these cases, since the recoil energy is negligible, the emitted gamma rays have the appropriate energy and resonance can occur.
In general (depending on the half-life of the decay), gamma rays have very narrow linewidths. This means they are very sensitive to small changes in the energies of nuclear transitions. In fact, gamma rays can be used as a probe to observe the effects of interactions between a nucleus and its electrons and those of its neighbors. This is the basis for Mössbauer spectroscopy, which combines the Mössbauer effect with the Doppler effect
to monitor such interactions.
Zero-phonon optical transitions
, a process closely analogous to the Mössbauer effect, can be observed in lattice-bound chromophore
s at low temperatures.
Atomic Recoil
Atomic recoil is the result of the interaction of an atom with an energetic elementary particle, when the momentum of the interacting particle is transferred to the atom as whole without altering non-translational degrees of freedom of the atom...
-free emission and absorption of γ radiation
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
by atomic nuclei bound in a solid. Its main application is in Mössbauer spectroscopy.
History
The emission and absorption of x-rayX-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
s by gases had been observed previously, and it was expected that a similar phenomenon would be found for gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s, which are created by nuclear
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
transitions (as opposed to x-rays, which are typically produced by electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
ic transitions). However, attempts to observe gamma-ray resonance in gases failed due to energy being lost to recoil, preventing resonance (the Doppler effect
Doppler effect
The Doppler effect , named after Austrian physicist Christian Doppler who proposed it in 1842 in Prague, is the change in frequency of a wave for an observer moving relative to the source of the wave. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from...
also broadens the gamma-ray spectrum). Mössbauer was able to observe resonance in solid iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
, which raised the question of why gamma-ray resonance was possible in solids, but not in gases. Mössbauer proposed that, for the case of atoms bound into a solid, under certain circumstances a fraction of the nuclear events could occur essentially without recoil. He attributed the observed resonance to this recoil-free fraction of nuclear events.
The Mössbauer effect was one of the last major discoveries in physics to be originally reported in German language. The first report in English language was a letter describing a repetition of the experiment.
The discovery was rewarded with the Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
in 1961 together with Robert Hofstadter
Robert Hofstadter
Robert Hofstadter was an American physicist. He was the joint winner of the 1961 Nobel Prize in Physics "for his pioneering studies of electron scattering in atomic nuclei and for his consequent discoveries concerning the structure of nucleons."-Biography :Born in New York City, he entered City...
's research of electron scattering
Electron scattering
Electron scattering is the process whereby an electron is deflected from its original trajectory. As they are charged particles, they are subject to electromagnetic forces.-Phenomena:...
in atomic nuclei.
Description

The amount of energy in the recoiling body can be found from momentum conservation:

where is the momentum of the recoiling matter, and the momentum of the gamma ray. Substituting energy into the equation gives:
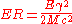
where ( for ) is the energy lost as recoil, is the energy of the gamma ray ( for ), ( for ) is the mass of the emitting or absorbing body, and c is the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
. In the case of a gas the emitting and absorbing bodies are atoms, so the mass is quite small, resulting in a large recoil energy, which prevents resonance. (Note that the same equation applies for recoil energy losses in x-rays, but the photon energy is much less, resulting in a lower energy loss, which is why gas-phase resonance could be observed with x-rays.)
In a solid, the nuclei are bound to the lattice and do not recoil in the same way as in a gas. The lattice as a whole recoils but the recoil energy is negligible because the in the above equation is the mass of the whole lattice. However, the energy in a decay can be taken up or supplied by lattice vibrations. The energy of these vibrations is quantised in units known as phonons. The Mössbauer effect occurs because there is a finite probability of a decay occurring involving no phonons. Thus in a fraction of the nuclear events (the recoil-free fraction, given by the Lamb–Mössbauer factor
Lamb–Mössbauer factor
In physics, the Lamb–Mössbauer factor or elastic incoherent structure factor is the ratio of elastic to total incoherent neutron scattering, or the ratio of recoil-free to total nuclear resonant absorption in Mössbauer spectroscopy...
), the entire crystal acts as the recoiling body, and these events are essentially recoil-free. In these cases, since the recoil energy is negligible, the emitted gamma rays have the appropriate energy and resonance can occur.
In general (depending on the half-life of the decay), gamma rays have very narrow linewidths. This means they are very sensitive to small changes in the energies of nuclear transitions. In fact, gamma rays can be used as a probe to observe the effects of interactions between a nucleus and its electrons and those of its neighbors. This is the basis for Mössbauer spectroscopy, which combines the Mössbauer effect with the Doppler effect
Doppler effect
The Doppler effect , named after Austrian physicist Christian Doppler who proposed it in 1842 in Prague, is the change in frequency of a wave for an observer moving relative to the source of the wave. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from...
to monitor such interactions.
Zero-phonon optical transitions
Zero-phonon line and phonon sideband
The zero-phonon line and the phonon sideband jointly constitute the line shape of individual light absorbing and emitting molecules embedded into a transparent solid matrix. When the host matrix contains many chromophores, each will contribute a zero-phonon line and a phonon sideband to the...
, a process closely analogous to the Mössbauer effect, can be observed in lattice-bound chromophore
Chromophore
A chromophore is the part of a molecule responsible for its color. The color arises when a molecule absorbs certain wavelengths of visible light and transmits or reflects others. The chromophore is a region in the molecule where the energy difference between two different molecular orbitals falls...
s at low temperatures.

