
Iridium
Encyclopedia
Iridium is the chemical element
with atomic number
77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal
of the platinum family
, iridium is the second-densest
element (after osmium
) and is the most corrosion
-resistant metal, even at temperatures as high as 2000 °C. Although only certain molten salts and halogen
s are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable.
Iridium was discovered in 1803 among insoluble impurities in natural platinum
. Smithson Tennant
, the primary discoverer, named the iridium for the goddess Iris
, personification of the rainbow, because of the striking and diverse colors of its salts. Iridium is one of the rarest elements
in the Earth's crust, with annual production and consumption of only three tonne
s. and are the only two naturally occurring isotope
s of iridium as well as the only stable isotope
s; the latter is the more abundant of the two.
The most important iridium compounds in use are the salts and acids it forms with chlorine
, though iridium also forms a number of organometallic compounds used in industrial catalysis
, and in research. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-end spark plug
s, crucible
s for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process
. Iridium radioisotopes are used in some radioisotope thermoelectric generator
s.
The unusually high abundance of iridium in the clay layer at the K–T geologic boundary
gave rise to the Alvarez hypothesis
that the impact of a massive extraterrestrial object caused the extinction of dinosaurs and many other species 65 million years ago. Iridium is found in meteorites with an abundance much higher than its average abundance in the Earth's crust. It is thought that the total amount of iridium in the planet Earth is much higher than that observed in crustal rocks, but as with other platinum group metals, the high density and tendency of iridium to bond with iron caused most iridium to descend below the crust when the planet was young and still molten.
 A member of the platinum group
A member of the platinum group
metal
s, iridium is white, resembling platinum
, but with a slight yellowish cast. Because of its hardness, brittleness, and very high melting point (the ninth highest of all elements), solid iridium is difficult to machine, form, or work, and thus powder metallurgy
is commonly employed instead. It is the only metal to maintain good mechanical properties in air at temperatures above 1600 °C. Iridium has a very high boiling point (10th among all elements) and becomes a superconductor at temperatures below 0.14 K
.
Iridium's modulus of elasticity is the second highest among the metals, only being surpassed by osmium
. This, together with a high modulus of rigidity and a very low figure for Poisson's ratio
(the relationship of longitudinal to lateral strain
), indicate the high degree of stiffness
and resistance to deformation that have rendered its fabrication into useful components a matter of great difficulty. Despite these limitations and iridium's high cost, a number of applications have developed where mechanical strength is an essential factor in some of the extremely severe conditions encountered in modern technology.
The measured density
of iridium is only slightly lower (by about 0.12%) than that of osmium
, the densest element known. There had been some ambiguity regarding which of the two elements was denser, due to the small size of the difference in density and difficulties in measuring it accurately, but, with increased accuracy in factors used for calculating density X-ray crystallographic
data yielded densities of 22.56 g/cm3 for iridium and 22.59 g/cm3 for osmium.
, aqua regia
, molten metals or silicates at high temperatures. It can, however, be attacked by some molten salts, such as sodium cyanide
and potassium cyanide
, as well as oxygen
and the halogen
s (particularly fluorine
) at higher temperatures.
Iridium forms compounds in oxidation state
s between −3 to +6; the most common oxidation states are +3 and +4. Well-characterized examples of the highest oxidation state are rare, but include
and two mixed oxides and .
Iridium dioxide
, , a brown powder, is the only well-characterized oxide of iridium. A sesquioxide
, , has been described as a blue-black powder which is oxidized to by . The corresponding disulfides, diselenides, sesquisulfides and sesquiselenides are known and has also been reported. Iridium also forms iridates with oxidation states +4 and +5, such as and , which can be prepared from the reaction of potassium oxide
or potassium superoxide
with iridium at high temperatures.
While no binary
hydride
s of iridium, are known, complexes are known that contain and , where iridium has the +1 and +3 oxidation states, respectively. The ternary hydride is believed to contain both the and the 18-electron anion.
No monohalides or dihalides are known, whereas trihalides, , are known for all of the halogens. For oxidation states +4 and above, only the tetrafluoride
, pentafluoride
and hexafluoride are known. Iridium hexafluoride, , is a volatile and highly reactive yellow solid, composed of octahedral molecules. It decomposes in water and is reduced to , a crystalline solid, by iridium black. Iridium pentafluoride has similar properties but it is actually a tetramer
, , formed by four corner-sharing octahedra.
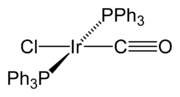 Hexachloroiridic(IV) acid, , and its ammonium salt are the most important iridium compounds from an industrial perspective. They are involved in the purification of iridium and used as precursors for most other iridium compounds, as well as in the preparation of anode
Hexachloroiridic(IV) acid, , and its ammonium salt are the most important iridium compounds from an industrial perspective. They are involved in the purification of iridium and used as precursors for most other iridium compounds, as well as in the preparation of anode
coatings. The ion has an intense dark brown color, and can be readily reduced to the lighter-colored and vice versa. Iridium trichloride
, , which can be obtained in anhydrous form from direct oxidation of iridium powder by chlorine
at 650 °C, or in hydrated form by dissolving in hydrochloric acid
, is often used as a starting material for the synthesis of other Ir(III) compounds. Another compound used as a starting material is ammonium hexachloroiridate(III), . Iridium(III) complexes are diamagnetic (low-spin) and generally have an octahedral molecular geometry
.
Organoiridium compound
s contain iridium–carbon
bonds where the metal is usually in lower oxidation states. For example, oxidation state zero is found in tetrairidium dodecacarbonyl
, , which is the most common and stable binary carbonyl
of iridium. In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex
, , which has the unusual property of binding to the dioxygen molecule, . Another one is Crabtree's catalyst
, a homogeneous catalyst for hydrogenation
reactions. These compounds are both square planar
, d8 complexes, with a total of 16 valence electron
s, which accounts for their reactivity.
s, 191Ir and 193Ir, with natural abundance
s of 37.3% and 62.7%, respectively. At least 34 radioisotopes have also been synthesized, ranging in mass number
from 164 to 199. 192Ir, which falls between the two stable isotopes, is the most stable radioisotope, with a half-life
of 73.827 days, and finds application in brachytherapy
and in industrial radiography
, particularly for non-destructive testing of welds in steel in the oil and gas industries; iridium-192 sources have been responsible for a number of radiological accidents. Three other isotopes have half-lives of at least a day—188Ir, 189Ir, 190Ir. Isotopes with masses below 191 decay by some combination of β+ decay, α decay
, and proton emission
, with the exceptions of 189Ir, which decays by electron capture
, and 190Ir, which decays by positron emission
. Synthetic isotopes heavier than 191 decay by β− decay, although 192Ir also has a minor electron capture
decay path. All known isotopes of iridium were discovered between 1934 and 2001; the most recent is 171Ir.
At least 32 metastable isomers
have been characterized, ranging in mass number from 164 to 197. The most stable of these is 192m2Ir, which decays by isomeric transition
with a half-life of 241 years, making it more stable than any of iridium's synthetic isotopes in their ground states. The least stable isomer is 190m3Ir with a half-life of only 2 µs. The isotope 191Ir was the first one of any element to be shown to present a Mössbauer effect
. This renders it useful for Mössbauer spectroscopy for research in physics, chemistry, biochemistry, metallurgy, and mineralogy.
. Native
platinum used by ancient Ethiopians and by South American cultures always contained a small amount of the other platinum group metals, including iridium. Platinum reached Europe as platina ("small silver"), found in the 17th century by the Spanish conquerors in a region today known as the department of Chocó in Colombia
. The discovery that this metal was not an alloy of known elements, but instead a distinct new element, did not occur until 1748.
Chemists who studied platinum dissolved it in aqua regia
(a mixture of hydrochloric
and nitric acid
s) to create soluble salts. They always observed a small amount of a dark, insoluble residue. Joseph Louis Proust thought that the residue was graphite
. The French chemists Victor Collet-Descotils, Antoine François, comte de Fourcroy
, and Louis Nicolas Vauquelin
also observed the black residue in 1803, but did not obtain enough for further experiments.
In 1803, British scientist Smithson Tennant
(1761–1815) analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin treated the powder alternately with alkali and acids and obtained a volatile new oxide, which he believed to be of this new metal—which he named ptene, from the Greek word (ptènos) for winged. Tennant, who had the advantage of a much greater amount of residue, continued his research and identified the two previously undiscovered elements in the black residue, iridium and osmium. He obtained dark red crystals (probably of ]·n) by a sequence of reactions with sodium hydroxide and hydrochloric acid
. He named iridium after Iris
, the Greek winged goddess of the rainbow and the messenger of the Olympian gods, because many of the salts he obtained were strongly colored.Iridium literally means "of rainbows". Discovery of the new elements was documented in a letter to the Royal Society
on June 21, 1804.
 British scientist John George Children
British scientist John George Children
was the first to melt a sample of iridium in 1813 with the aid of "the greatest galvanic battery that has ever been constructed" (at that time). The first to obtain high purity iridium was Robert Hare
in 1842. He found that it had a density of around 21.8 g/cm3 and noted that the metal is nearly unmalleable and very hard. The first melting in appreciable quantity was done by Henri Sainte-Claire Deville and Jules Henri Debray in 1860. They required burning more than 300 L of pure and for each kilogram of iridium.
These extreme difficulties in melting the metal limited the possibilities for handling iridium. John Isaac Hawkins
was looking to obtain a fine and hard point for fountain pen nibs and in 1834 managed to create an iridium-pointed gold pen. In 1880 John Holland
and William Lofland Dudley were able to melt iridium by adding phosphorus
and patented the process in the United States; British company Johnson Matthey
later stated that they had been using a similar process since 1837 and had already presented fused iridium at a number of World Fairs. The first use of an alloy of iridium with ruthenium in thermocouple
s was made by Otto Feussner in 1933. These allowed for the measurement of high temperatures in air up to 2000 °C.
In 1957 Rudolf Mössbauer, in what has been called one of the "landmark experiments in twentieth century physics", discovered the resonant and recoil
-free emission and absorption of gamma ray
s by atoms in a solid metal sample containing only 191Ir. This phenomenon, known as the Mössbauer effect
(which has since been observed for other nuclei, such as 57Fe), and developed as Mössbauer spectroscopy, has made important contributions to research in physics, chemistry, biochemistry, metallurgy, and mineralogy. Mössbauer received the Nobel Prize in Physics
in 1961, just three years after he published his discovery.
 Iridium is one of the least abundant elements
Iridium is one of the least abundant elements
in the Earth's crust
, having an average mass fraction of 0.001 ppm in crustal rock; gold
is 40 times more abundant, platinum
is 10 times more abundant, and silver
and mercury
are 80 times more abundant. Tellurium is about as abundant as iridium, and only three naturally occurring elements are less abundant: rhenium
, ruthenium
, and rhodium
, iridium being 10 times more abundant than the last two. In contrast to its low abundance in crustal rock, iridium is relatively common in meteorite
s, with concentrations of 0.5 ppm or more. It is thought that the overall concentration of iridium on Earth is much higher than what is observed in crustal rocks, but because of the density and siderophilic
("iron-loving") character of iridium, it descended below the crust and into the Earth's core
when the planet was still molten.
Iridium is found in nature as an uncombined element or in natural alloy
s; especially the iridium–osmium alloys, osmiridium
(osmium rich), and iridiosmium (iridium rich). In the nickel
and copper
deposits the platinum group metals occur as sulfide
s (i.e. (Pt,Pd)S), tellurides
(i.e. PtBiTe), antimonide
s (PdSb), and arsenide
s (i.e. ). In all of these compounds platinum is exchanged by a small amount of iridium and osmium. As with all of the platinum group metals, iridium can be found naturally in alloys with raw nickel or raw copper
.
Within the Earth's crust, iridium is found at highest concentrations in three types of geologic structure: igneous deposits (crustal intrusions from below), impact craters, and deposits reworked from one of the former structures. The largest known primary reserves are in the Bushveld igneous complex
in South Africa
, though the large copper–nickel deposits near Norilsk in Russia
, and the Sudbury Basin
in Canada
are also significant sources of iridium. Smaller reserves are found in the United States. Iridium is also found in secondary deposits, combined with platinum and other platinum group metals in alluvial
deposits. The alluvial deposits used by pre-Columbian
people in the Chocó Department
of Colombia
are still a source for platinum-group metals. As of 2003 the world reserves had not been estimated.

The K–T boundary
of 65 million years ago, marking the temporal border between the Cretaceous
and Tertiary
periods of geological time
, was identified by a thin stratum
of iridium-rich clay
. A team led by Luis Alvarez proposed in 1980 an extraterrestrial origin for this iridium, attributing it to an asteroid
or comet
impact. Their theory, known as the Alvarez hypothesis
, is now widely accepted to explain the demise of the dinosaur
s. A large buried impact crater structure with an estimated age of about 65 million years was later identified under what is now the Yucatán Peninsula
(the Chicxulub crater
). Dewey M. McLean and others argue that the iridium may have been of volcanic
origin instead, as the Earth
's core is rich in iridium, and active volcanoes such as Piton de la Fournaise
, in the island of Réunion
, are still releasing iridium.
/ozt
)
|-
|2001||415.25
|-
|2002||294.62
|-
|2003||93.02
|-
|2004||185.33
|-
|2005||169.51
|-
|2006||349.45
|-
|2007||444.43
|-
|2008||448.34
|-
|2009||420.4
|-
|2010||635
|}
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
of the platinum family
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
, iridium is the second-densest
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
element (after osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
) and is the most corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
-resistant metal, even at temperatures as high as 2000 °C. Although only certain molten salts and halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable.
Iridium was discovered in 1803 among insoluble impurities in natural platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
. Smithson Tennant
Smithson Tennant
Smithson Tennant FRS was an English chemist.Tennant is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal. The mineral tennantite is...
, the primary discoverer, named the iridium for the goddess Iris
Iris (mythology)
In Greek mythology, Iris is the personification of the rainbow and messenger of the gods. As the sun unites Earth and heaven, Iris links the gods to humanity...
, personification of the rainbow, because of the striking and diverse colors of its salts. Iridium is one of the rarest elements
Abundance of elements in Earth's crust
The table shows the abundance of elements in Earth's crust. Numbers show percentage or parts per million in mass; 10,000 ppm = 1%.Note that numbers are estimates, and they will vary depending on source and method of estimation. Order of magnitude of data can roughly be relied upon.The table shows...
in the Earth's crust, with annual production and consumption of only three tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s. and are the only two naturally occurring isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of iridium as well as the only stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
s; the latter is the more abundant of the two.
The most important iridium compounds in use are the salts and acids it forms with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, though iridium also forms a number of organometallic compounds used in industrial catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, and in research. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-end spark plug
Spark plug
A spark plug is an electrical device that fits into the cylinder head of some internal combustion engines and ignites compressed fuels such as aerosol, gasoline, ethanol, and liquefied petroleum gas by means of an electric spark.Spark plugs have an insulated central electrode which is connected by...
s, crucible
Crucible
A crucible is a container used for metal, glass, and pigment production as well as a number of modern laboratory processes, which can withstand temperatures high enough to melt or otherwise alter its contents...
s for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process
Chloralkali process
The chloralkali process is an industrial process for the electrolysis of sodium chloride solution . Depending on the method several products beside hydrogen can be produced. If the products are separated, chlorine and sodium hydroxide are the products; by mixing, sodium hypochlorite or sodium...
. Iridium radioisotopes are used in some radioisotope thermoelectric generator
Radioisotope thermoelectric generator
A radioisotope thermoelectric generator is an electrical generator that obtains its power from radioactive decay. In such a device, the heat released by the decay of a suitable radioactive material is converted into electricity by the Seebeck effect using an array of thermocouples.RTGs can be...
s.
The unusually high abundance of iridium in the clay layer at the K–T geologic boundary
K–T boundary
The K–T boundary is a geological signature, usually a thin band, dated to 65.5 ± 0.3 Ma ago. K is the traditional abbreviation for the Cretaceous period, and T is the abbreviation for the Tertiary period...
gave rise to the Alvarez hypothesis
Alvarez hypothesis
The Alvarez hypothesis claims that the mass extinction of the dinosaurs and many other living things was caused by the impact of a large asteroid on the Earth sixty-five million years ago, called the Cretaceous-Tertiary extinction event. Evidence indicates that the asteroid fell in the Yucatán...
that the impact of a massive extraterrestrial object caused the extinction of dinosaurs and many other species 65 million years ago. Iridium is found in meteorites with an abundance much higher than its average abundance in the Earth's crust. It is thought that the total amount of iridium in the planet Earth is much higher than that observed in crustal rocks, but as with other platinum group metals, the high density and tendency of iridium to bond with iron caused most iridium to descend below the crust when the planet was young and still molten.
Physical properties

Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s, iridium is white, resembling platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, but with a slight yellowish cast. Because of its hardness, brittleness, and very high melting point (the ninth highest of all elements), solid iridium is difficult to machine, form, or work, and thus powder metallurgy
Powder metallurgy
Powder metallurgy is the process of blending fine powdered materials, pressing them into a desired shape , and then heating the compressed material in a controlled atmosphere to bond the material . The powder metallurgy process generally consists of four basic steps: powder manufacture, powder...
is commonly employed instead. It is the only metal to maintain good mechanical properties in air at temperatures above 1600 °C. Iridium has a very high boiling point (10th among all elements) and becomes a superconductor at temperatures below 0.14 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
.
Iridium's modulus of elasticity is the second highest among the metals, only being surpassed by osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
. This, together with a high modulus of rigidity and a very low figure for Poisson's ratio
Poisson's ratio
Poisson's ratio , named after Siméon Poisson, is the ratio, when a sample object is stretched, of the contraction or transverse strain , to the extension or axial strain ....
(the relationship of longitudinal to lateral strain
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
), indicate the high degree of stiffness
Stiffness
Stiffness is the resistance of an elastic body to deformation by an applied force along a given degree of freedom when a set of loading points and boundary conditions are prescribed on the elastic body.-Calculations:...
and resistance to deformation that have rendered its fabrication into useful components a matter of great difficulty. Despite these limitations and iridium's high cost, a number of applications have developed where mechanical strength is an essential factor in some of the extremely severe conditions encountered in modern technology.
The measured density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of iridium is only slightly lower (by about 0.12%) than that of osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
, the densest element known. There had been some ambiguity regarding which of the two elements was denser, due to the small size of the difference in density and difficulties in measuring it accurately, but, with increased accuracy in factors used for calculating density X-ray crystallographic
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
data yielded densities of 22.56 g/cm3 for iridium and 22.59 g/cm3 for osmium.
Chemical properties
Iridium is the most corrosion-resistant metal known: it is not attacked by almost any acidAcid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
, aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
, molten metals or silicates at high temperatures. It can, however, be attacked by some molten salts, such as sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
and potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
, as well as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s (particularly fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
) at higher temperatures.
Compounds
| Oxidation statesMost common oxidation states of iridium are in bold. The right column lists one representative compound for each oxidation state. | |
|---|---|
| −3 | |
| −1 | |
| 0 | |
| +1 | |
| +2 | |
| +3 | |
| +4 | |
| +5 | |
| +6 | |
Iridium forms compounds in oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s between −3 to +6; the most common oxidation states are +3 and +4. Well-characterized examples of the highest oxidation state are rare, but include
Iridium(VI) fluoride
Iridium fluoride is a volatile and highly reactive yellow solid, with an octahedral molecular structure . It is one of only a few compounds with iridium in its highest oxidation state, +6...
and two mixed oxides and .
Iridium dioxide
Iridium(IV) oxide
Iridium oxide, IrO2, is the only well characterised oxide of iridium. Its crystal has the TiO2, rutile structure containing six coordinate iridium and three coordinate oxygen....
, , a brown powder, is the only well-characterized oxide of iridium. A sesquioxide
Sesquioxide
A sesquioxide is an oxide containing three atoms of oxygen with two atoms of another element. For example, aluminium oxide is a sesquioxide.Many sesquioxides contain the metal in the +3 oxidation state and the oxide ion, e.g., Al2O3, La2O3...
, , has been described as a blue-black powder which is oxidized to by . The corresponding disulfides, diselenides, sesquisulfides and sesquiselenides are known and has also been reported. Iridium also forms iridates with oxidation states +4 and +5, such as and , which can be prepared from the reaction of potassium oxide
Potassium oxide
Potassium oxide is an ionic compound of potassium and oxygen. This pale yellow solid, the simplest oxide of potassium, is a rarely encountered, highly reactive compound...
or potassium superoxide
Potassium superoxide
Potassium superoxide is the chemical compound with the formula KO2. This rare salt of the superoxide ion is produced by burning molten potassium in pure oxygen...
with iridium at high temperatures.
While no binary
Binary compound
A binary compound is a chemical compound that contains exactly two different elements. Examples of binary ionic compounds include calcium chloride , sodium fluoride , and magnesium oxide , whilst examples of binary covalent compounds include water , carbon monoxide , and sulfur hexafluoride...
hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
s of iridium, are known, complexes are known that contain and , where iridium has the +1 and +3 oxidation states, respectively. The ternary hydride is believed to contain both the and the 18-electron anion.
No monohalides or dihalides are known, whereas trihalides, , are known for all of the halogens. For oxidation states +4 and above, only the tetrafluoride
Iridium(IV) fluoride
Iridium fluoride is a chemical compound of iridium and fluorine, with the chemical formula IrF4 and is a dark brown solid. Early reports of IrF4 prior to 1965 are questionable and appear to describe the compound IrF5...
, pentafluoride
Iridium(V) fluoride
Iridium fluoride, IrF5, is a chemical compound of iridium and fluorine, first described by Neil Bartlett in 1965. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms. This structure is shared with RuF5 and OsF5...
and hexafluoride are known. Iridium hexafluoride, , is a volatile and highly reactive yellow solid, composed of octahedral molecules. It decomposes in water and is reduced to , a crystalline solid, by iridium black. Iridium pentafluoride has similar properties but it is actually a tetramer
Tetramer
A tetramer is a protein with four subunits . There are homotetramers such as glutathione S-transferase or single-strand binding protein, dimers of hetero-dimers such as hemoglobin , and heterotetramers, where each subunit is different.-Subunit interactions in tetramers:The interactions between...
, , formed by four corner-sharing octahedra.

Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
coatings. The ion has an intense dark brown color, and can be readily reduced to the lighter-colored and vice versa. Iridium trichloride
Iridium(III) chloride
Iridium chloride is the inorganic compound with the formula IrCl3. This material is relatively rare, but the related hydrate is useful for preparing other iridium compounds. The anhydrous salt is a dark green crystalline solid...
, , which can be obtained in anhydrous form from direct oxidation of iridium powder by chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
at 650 °C, or in hydrated form by dissolving in hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, is often used as a starting material for the synthesis of other Ir(III) compounds. Another compound used as a starting material is ammonium hexachloroiridate(III), . Iridium(III) complexes are diamagnetic (low-spin) and generally have an octahedral molecular geometry
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
.
Organoiridium compound
Organoiridium compound
Organoiridium compounds contain iridium-carbon chemical bonds. Compounds with Ir-C bonds are found in oxidation states from 0 to V. For example, oxidation state zero is found in tetrairidium dodecacarbonyl, , which is the most common and stable binary carbonyl of iridium. In this compound, each of...
s contain iridium–carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bonds where the metal is usually in lower oxidation states. For example, oxidation state zero is found in tetrairidium dodecacarbonyl
Tetrairidium dodecacarbonyl
Tetrairidium dodecacarbonyl is the chemical compound with the formula Ir412. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents. It has been used to prepare bimetallic clusters and catalysts,...
, , which is the most common and stable binary carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
of iridium. In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex
Vaska's complex
Vaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
, , which has the unusual property of binding to the dioxygen molecule, . Another one is Crabtree's catalyst
Crabtree's catalyst
Crabtree's catalyst is the name given to a complex of iridium with 1,5-cyclooctadiene, tris-cyclohexylphosphine, and pyridine. It is a homogeneous catalyst for hydrogenation reactions, developed by Robert H. Crabtree, a professor at Yale University...
, a homogeneous catalyst for hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
reactions. These compounds are both square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
, d8 complexes, with a total of 16 valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s, which accounts for their reactivity.
Isotopes
Iridium has two naturally occurring, stable isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, 191Ir and 193Ir, with natural abundance
Natural abundance
In chemistry, natural abundance refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table...
s of 37.3% and 62.7%, respectively. At least 34 radioisotopes have also been synthesized, ranging in mass number
Mass number
The mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
from 164 to 199. 192Ir, which falls between the two stable isotopes, is the most stable radioisotope, with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 73.827 days, and finds application in brachytherapy
Brachytherapy
Brachytherapy , also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment...
and in industrial radiography
Radiography
Radiography is the use of X-rays to view a non-uniformly composed material such as the human body. By using the physical properties of the ray an image can be developed which displays areas of different density and composition....
, particularly for non-destructive testing of welds in steel in the oil and gas industries; iridium-192 sources have been responsible for a number of radiological accidents. Three other isotopes have half-lives of at least a day—188Ir, 189Ir, 190Ir. Isotopes with masses below 191 decay by some combination of β+ decay, α decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
, and proton emission
Proton emission
Proton emission is a type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state of very...
, with the exceptions of 189Ir, which decays by electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
, and 190Ir, which decays by positron emission
Positron emission
Positron emission or beta plus decay is a type of beta decay in which a proton is converted, via the weak force, to a neutron, releasing a positron and a neutrino....
. Synthetic isotopes heavier than 191 decay by β− decay, although 192Ir also has a minor electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
decay path. All known isotopes of iridium were discovered between 1934 and 2001; the most recent is 171Ir.
At least 32 metastable isomers
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
have been characterized, ranging in mass number from 164 to 197. The most stable of these is 192m2Ir, which decays by isomeric transition
Isomeric transition
An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer....
with a half-life of 241 years, making it more stable than any of iridium's synthetic isotopes in their ground states. The least stable isomer is 190m3Ir with a half-life of only 2 µs. The isotope 191Ir was the first one of any element to be shown to present a Mössbauer effect
Mössbauer effect
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of γ radiation by atomic nuclei bound in a solid...
. This renders it useful for Mössbauer spectroscopy for research in physics, chemistry, biochemistry, metallurgy, and mineralogy.
History
The discovery of iridium is intertwined with that of platinum and the other metals of the platinum groupPlatinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
. Native
Native Metal
A native metal is any metal that is found in its metallic form, either pure or as an alloy, in nature. Metals that can be found as native deposits singly and/or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, nickel, selenium, tantalum, tellurium,...
platinum used by ancient Ethiopians and by South American cultures always contained a small amount of the other platinum group metals, including iridium. Platinum reached Europe as platina ("small silver"), found in the 17th century by the Spanish conquerors in a region today known as the department of Chocó in Colombia
Colombia
Colombia, officially the Republic of Colombia , is a unitary constitutional republic comprising thirty-two departments. The country is located in northwestern South America, bordered to the east by Venezuela and Brazil; to the south by Ecuador and Peru; to the north by the Caribbean Sea; to the...
. The discovery that this metal was not an alloy of known elements, but instead a distinct new element, did not occur until 1748.
Chemists who studied platinum dissolved it in aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
(a mixture of hydrochloric
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
s) to create soluble salts. They always observed a small amount of a dark, insoluble residue. Joseph Louis Proust thought that the residue was graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. The French chemists Victor Collet-Descotils, Antoine François, comte de Fourcroy
Antoine François, comte de Fourcroy
Antoine François, comte de Fourcroy was a French chemist and a contemporary of Antoine Lavoisier. Fourcroy collaborated with Lavoisier, Guyton de Morveau, and Claude Berthollet on the Méthode de nomenclature chimique, a work that helped standardize chemical nomenclature.-Life and work:Fourcroy...
, and Louis Nicolas Vauquelin
Louis Nicolas Vauquelin
Nicolas Louis Vauquelin , was a French pharmacist and chemist.-Early life:Vauquelin was born at Saint-André-d'Hébertot in Normandy, France. His first acquaintance with chemistry was gained as laboratory assistant to an apothecary in Rouen , and after various vicissitudes he obtained an introduction...
also observed the black residue in 1803, but did not obtain enough for further experiments.
In 1803, British scientist Smithson Tennant
Smithson Tennant
Smithson Tennant FRS was an English chemist.Tennant is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal. The mineral tennantite is...
(1761–1815) analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin treated the powder alternately with alkali and acids and obtained a volatile new oxide, which he believed to be of this new metal—which he named ptene, from the Greek word (ptènos) for winged. Tennant, who had the advantage of a much greater amount of residue, continued his research and identified the two previously undiscovered elements in the black residue, iridium and osmium. He obtained dark red crystals (probably of ]·n) by a sequence of reactions with sodium hydroxide and hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. He named iridium after Iris
Iris (mythology)
In Greek mythology, Iris is the personification of the rainbow and messenger of the gods. As the sun unites Earth and heaven, Iris links the gods to humanity...
, the Greek winged goddess of the rainbow and the messenger of the Olympian gods, because many of the salts he obtained were strongly colored.Iridium literally means "of rainbows". Discovery of the new elements was documented in a letter to the Royal Society
Royal Society
The Royal Society of London for Improving Natural Knowledge, known simply as the Royal Society, is a learned society for science, and is possibly the oldest such society in existence. Founded in November 1660, it was granted a Royal Charter by King Charles II as the "Royal Society of London"...
on June 21, 1804.

John George Children
John George Children was a British chemist, mineralogist and zoologist.Children studied at Queens' College, Cambridge. In 1822 he was working as a librarian in the Department of Antiquities at the British Museum when he was appointed assistant keeper of the Natural History Department in succession...
was the first to melt a sample of iridium in 1813 with the aid of "the greatest galvanic battery that has ever been constructed" (at that time). The first to obtain high purity iridium was Robert Hare
Robert Hare (chemist)
Robert Hare was an early American chemist.Hare was born in Philadelphia, Pennsylvania on January 17, 1781. He developed and experimented with the oxy-hydrogen blowpipe, with Edward Daniel Clarke of Oxford, shortly after 1800. He married Harriett Clark and had six children...
in 1842. He found that it had a density of around 21.8 g/cm3 and noted that the metal is nearly unmalleable and very hard. The first melting in appreciable quantity was done by Henri Sainte-Claire Deville and Jules Henri Debray in 1860. They required burning more than 300 L of pure and for each kilogram of iridium.
These extreme difficulties in melting the metal limited the possibilities for handling iridium. John Isaac Hawkins
John Isaac Hawkins
John Isaac Hawkins was an inventor who practiced civil engineering.He was known as the co-inventor of the ever-pointed pencil, an early mechanical pencil, and of the upright piano.-Life:...
was looking to obtain a fine and hard point for fountain pen nibs and in 1834 managed to create an iridium-pointed gold pen. In 1880 John Holland
John Holland (pen maker)
John Holland was a prominent businessman and industrialist whose "John Holland Gold Pen Company" was a large maker of pens and related products during the late 19th century...
and William Lofland Dudley were able to melt iridium by adding phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and patented the process in the United States; British company Johnson Matthey
Johnson Matthey
Johnson Matthey plc is multinational chemicals and precious metals company headquartered in London, United Kingdom.It is listed on the London Stock Exchange and is a constituent of the FTSE 100 Index.-History:...
later stated that they had been using a similar process since 1837 and had already presented fused iridium at a number of World Fairs. The first use of an alloy of iridium with ruthenium in thermocouple
Thermocouple
A thermocouple is a device consisting of two different conductors that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a...
s was made by Otto Feussner in 1933. These allowed for the measurement of high temperatures in air up to 2000 °C.
In 1957 Rudolf Mössbauer, in what has been called one of the "landmark experiments in twentieth century physics", discovered the resonant and recoil
Recoil
Recoil is the backward momentum of a gun when it is discharged. In technical terms, the recoil caused by the gun exactly balances the forward momentum of the projectile and exhaust gasses, according to Newton's third law...
-free emission and absorption of gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s by atoms in a solid metal sample containing only 191Ir. This phenomenon, known as the Mössbauer effect
Mössbauer effect
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of γ radiation by atomic nuclei bound in a solid...
(which has since been observed for other nuclei, such as 57Fe), and developed as Mössbauer spectroscopy, has made important contributions to research in physics, chemistry, biochemistry, metallurgy, and mineralogy. Mössbauer received the Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
in 1961, just three years after he published his discovery.
Occurrence

Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
in the Earth's crust
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
, having an average mass fraction of 0.001 ppm in crustal rock; gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
is 40 times more abundant, platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
is 10 times more abundant, and silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
and mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
are 80 times more abundant. Tellurium is about as abundant as iridium, and only three naturally occurring elements are less abundant: rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
, and rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
, iridium being 10 times more abundant than the last two. In contrast to its low abundance in crustal rock, iridium is relatively common in meteorite
Meteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
s, with concentrations of 0.5 ppm or more. It is thought that the overall concentration of iridium on Earth is much higher than what is observed in crustal rocks, but because of the density and siderophilic
Goldschmidt classification
The Goldschmidt classification, developed by Victor Goldschmidt, is a geochemical classification which groups the chemical elements according to their preferred host phases into lithophile , siderophile , chalcophile , and atmophile .Some elements have affinities to more than one phase...
("iron-loving") character of iridium, it descended below the crust and into the Earth's core
Inner core
The inner core of the Earth, its innermost hottest part as detected by seismological studies, is a primarily solid ball about in radius, or about 70% that of the Moon...
when the planet was still molten.
Iridium is found in nature as an uncombined element or in natural alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s; especially the iridium–osmium alloys, osmiridium
Osmiridium
Osmiridium, are names given to natural alloys of osmium and iridium, with traces of other platinum group metals. Osmiridium has been defined as containing a higher proportion of iridium, while iridosmine contains more osmium...
(osmium rich), and iridiosmium (iridium rich). In the nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
deposits the platinum group metals occur as sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s (i.e. (Pt,Pd)S), tellurides
Telluride (chemistry)
The telluride ion is Te2−. It is the final stable member of the series of dianions O2−, S2−, and Se2− ....
(i.e. PtBiTe), antimonide
Antimonide
Antimonides are compounds of antimony with more electropositive elements. The antimonide ion is Sb3−.Many of them are flammable or decomposed by oxygen when heated since the antimonide ion is a reducing agent....
s (PdSb), and arsenide
Arsenide
Arsenide is an arsenic anion with the charge −3. The trianion is formed by the reduction of arsenic by three electrons. For example heating arsenic powder with excess sodium gives sodium arsenide . The anions have no existence in solution since they are extremely basic...
s (i.e. ). In all of these compounds platinum is exchanged by a small amount of iridium and osmium. As with all of the platinum group metals, iridium can be found naturally in alloys with raw nickel or raw copper
Native copper
Copper, as native copper, is one of the few metallic elements to occur in uncombined form as a natural mineral, although most commonly occurs in oxidized states and mixed with other elements...
.
Within the Earth's crust, iridium is found at highest concentrations in three types of geologic structure: igneous deposits (crustal intrusions from below), impact craters, and deposits reworked from one of the former structures. The largest known primary reserves are in the Bushveld igneous complex
Bushveld igneous complex
The Bushveld Igneous Complex is a large layered igneous intrusion within the Earth's crust which has been tilted and eroded and now outcrops around what appears to be the edge of a great geological basin, the Transvaal Basin. Located in South Africa, the BIC contains some of the richest ore...
in South Africa
South Africa
The Republic of South Africa is a country in southern Africa. Located at the southern tip of Africa, it is divided into nine provinces, with of coastline on the Atlantic and Indian oceans...
, though the large copper–nickel deposits near Norilsk in Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
, and the Sudbury Basin
Sudbury Basin
The Sudbury Basin, also known as Sudbury Structure or the Sudbury Nickel Irruptive, is a major geologic structure in Ontario, Canada. It is the second-largest known impact crater or astrobleme on Earth, as well as one of the oldest....
in Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
are also significant sources of iridium. Smaller reserves are found in the United States. Iridium is also found in secondary deposits, combined with platinum and other platinum group metals in alluvial
Alluvium
Alluvium is loose, unconsolidated soil or sediments, eroded, deposited, and reshaped by water in some form in a non-marine setting. Alluvium is typically made up of a variety of materials, including fine particles of silt and clay and larger particles of sand and gravel...
deposits. The alluvial deposits used by pre-Columbian
Pre-Columbian
The pre-Columbian era incorporates all period subdivisions in the history and prehistory of the Americas before the appearance of significant European influences on the American continents, spanning the time of the original settlement in the Upper Paleolithic period to European colonization during...
people in the Chocó Department
Chocó Department
Chocó is a department of Colombia known for its large Afro-Colombian population. It is in the west of the country, and is the only Colombian department to have coastlines on both the Pacific Ocean and the Atlantic Ocean. It also has all of Colombia's border with Panama. Its capital is...
of Colombia
Colombia
Colombia, officially the Republic of Colombia , is a unitary constitutional republic comprising thirty-two departments. The country is located in northwestern South America, bordered to the east by Venezuela and Brazil; to the south by Ecuador and Peru; to the north by the Caribbean Sea; to the...
are still a source for platinum-group metals. As of 2003 the world reserves had not been estimated.
K–T boundary presence

The K–T boundary
K–T boundary
The K–T boundary is a geological signature, usually a thin band, dated to 65.5 ± 0.3 Ma ago. K is the traditional abbreviation for the Cretaceous period, and T is the abbreviation for the Tertiary period...
of 65 million years ago, marking the temporal border between the Cretaceous
Cretaceous
The Cretaceous , derived from the Latin "creta" , usually abbreviated K for its German translation Kreide , is a geologic period and system from circa to million years ago. In the geologic timescale, the Cretaceous follows the Jurassic period and is followed by the Paleogene period of the...
and Tertiary
Tertiary
The Tertiary is a deprecated term for a geologic period 65 million to 2.6 million years ago. The Tertiary covered the time span between the superseded Secondary period and the Quaternary...
periods of geological time
Geologic time scale
The geologic time scale provides a system of chronologic measurement relating stratigraphy to time that is used by geologists, paleontologists and other earth scientists to describe the timing and relationships between events that have occurred during the history of the Earth...
, was identified by a thin stratum
Stratum
In geology and related fields, a stratum is a layer of sedimentary rock or soil with internally consistent characteristics that distinguish it from other layers...
of iridium-rich clay
Iridium anomaly
The term iridium anomaly commonly refers to an unusual abundance of the chemical element iridium in a layer of rock strata, often taken as evidence of an extraterrestrial impact event because of the case of such an anomaly at the Cretaceous–Tertiary boundary...
. A team led by Luis Alvarez proposed in 1980 an extraterrestrial origin for this iridium, attributing it to an asteroid
Asteroid
Asteroids are a class of small Solar System bodies in orbit around the Sun. They have also been called planetoids, especially the larger ones...
or comet
Comet
A comet is an icy small Solar System body that, when close enough to the Sun, displays a visible coma and sometimes also a tail. These phenomena are both due to the effects of solar radiation and the solar wind upon the nucleus of the comet...
impact. Their theory, known as the Alvarez hypothesis
Alvarez hypothesis
The Alvarez hypothesis claims that the mass extinction of the dinosaurs and many other living things was caused by the impact of a large asteroid on the Earth sixty-five million years ago, called the Cretaceous-Tertiary extinction event. Evidence indicates that the asteroid fell in the Yucatán...
, is now widely accepted to explain the demise of the dinosaur
Dinosaur
Dinosaurs are a diverse group of animals of the clade and superorder Dinosauria. They were the dominant terrestrial vertebrates for over 160 million years, from the late Triassic period until the end of the Cretaceous , when the Cretaceous–Paleogene extinction event led to the extinction of...
s. A large buried impact crater structure with an estimated age of about 65 million years was later identified under what is now the Yucatán Peninsula
Yucatán Peninsula
The Yucatán Peninsula, in southeastern Mexico, separates the Caribbean Sea from the Gulf of Mexico, with the northern coastline on the Yucatán Channel...
(the Chicxulub crater
Chicxulub Crater
The Chicxulub crater is an ancient impact crater buried underneath the Yucatán Peninsula in Mexico. Its center is located near the town of Chicxulub, after which the crater is named...
). Dewey M. McLean and others argue that the iridium may have been of volcanic
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
origin instead, as the Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
's core is rich in iridium, and active volcanoes such as Piton de la Fournaise
Piton de la Fournaise
Piton de la Fournaise : "Peak of the Furnace" is a shield volcano on the eastern side of Réunion island in the Indian Ocean. It is currently one of the most active volcanoes in the world, along with Kīlauea in the Hawaiian Islands , Stromboli, Etna and Mount Erebus in Antarctica...
, in the island of Réunion
Réunion
Réunion is a French island with a population of about 800,000 located in the Indian Ocean, east of Madagascar, about south west of Mauritius, the nearest island.Administratively, Réunion is one of the overseas departments of France...
, are still releasing iridium.
Production
($United States dollar
The United States dollar , also referred to as the American dollar, is the official currency of the United States of America. It is divided into 100 smaller units called cents or pennies....
/ozt
Troy ounce
The troy ounce is a unit of imperial measure. In the present day it is most commonly used to gauge the weight of precious metals. One troy ounce is nowadays defined as exactly 0.0311034768 kg = 31.1034768 g. There are approximately 32.1507466 troy oz in 1 kg...
)
|-
|2001||415.25
|-
|2002||294.62
|-
|2003||93.02
|-
|2004||185.33
|-
|2005||169.51
|-
|2006||349.45
|-
|2007||444.43
|-
|2008||448.34
|-
|2009||420.4
|-
|2010||635
|}
Iridium is obtained commercially as a by-product from nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
mining and processing. During electrorefining of copper and nickel, noble metals such as silver, gold and the platinum group metals as well as selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
and tellurium settle to the bottom of the cell as anode mud, which forms the starting point for their extraction. In order to separate the metals, they must first be brought into solution. Several methods are available depending on the separation process and the composition of the mixture; two representative methods are fusion with sodium peroxide
Sodium peroxide
Sodium peroxide is the inorganic compound with the formula Na2O2. This solid is the product when sodium is burned with oxygen. It is a strong base and a potent oxidizing agent. It exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and...
followed by dissolution in aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
, and dissolution in a mixture of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
with hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
.
After it is dissolved, iridium is separated from the other platinum group metals by precipitating or by extracting with organic amines. The first method is similar to the procedure Tennant and Wollaston used for their separation. The second method can be planned as continuous liquid–liquid extraction and is therefore more suitable for industrial scale production. In either case, the product is reduced using hydrogen, yielding the metal as a powder or sponge that can be treated using powder metallurgy
Powder metallurgy
Powder metallurgy is the process of blending fine powdered materials, pressing them into a desired shape , and then heating the compressed material in a controlled atmosphere to bond the material . The powder metallurgy process generally consists of four basic steps: powder manufacture, powder...
techniques.
Annual production of iridium circa 2000 was around 3 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s or about 100,000 troy ounce
Troy ounce
The troy ounce is a unit of imperial measure. In the present day it is most commonly used to gauge the weight of precious metals. One troy ounce is nowadays defined as exactly 0.0311034768 kg = 31.1034768 g. There are approximately 32.1507466 troy oz in 1 kg...
s (ozt).Like other precious metals, iridium is customarily traded in troy ounces, which are equivalent to about 31.1 grams. The price of iridium as of 2007 was $440 USD/ozt, but the price fluctuates considerably, as shown in the table. The high volatility
Volatility (finance)
In finance, volatility is a measure for variation of price of a financial instrument over time. Historic volatility is derived from time series of past market prices...
of the prices of the platinum group metals has been attributed to supply, demand, speculation
Speculation
In finance, speculation is a financial action that does not promise safety of the initial investment along with the return on the principal sum...
, and hoarding, amplified by the small size of the market and instability in the producing countries.
Applications
The global demand for iridium in 2007 was 119,000 troy ounceTroy ounce
The troy ounce is a unit of imperial measure. In the present day it is most commonly used to gauge the weight of precious metals. One troy ounce is nowadays defined as exactly 0.0311034768 kg = 31.1034768 g. There are approximately 32.1507466 troy oz in 1 kg...
s (3,700 kg), out of which 25,000 ozt (780 kg) were used for electrical applications such as spark plugs; 34,000 ozt (1,100 kg) for electrochemical applications such as electrodes for the chloralkali process
Chloralkali process
The chloralkali process is an industrial process for the electrolysis of sodium chloride solution . Depending on the method several products beside hydrogen can be produced. If the products are separated, chlorine and sodium hydroxide are the products; by mixing, sodium hypochlorite or sodium...
; 24,000 ozt (750 kg) for catalysis; and 36,000 ozt (1,100 kg) for other uses.
Industrial and medical
3.png)
Spinneret (polymers)
Spinneret refers to a multi-pored device through which a plastic polymer melt is extruded to form fibers. Streams of viscous polymer usually exit into cool air or liquid to solidify. The individual polymer chains tend to align in the fiber because of viscous flow. This airstream liquid-to-fiber...
, through which a plastic polymer melt is extruded to form fibers, such as rayon
Rayon
Rayon is a manufactured regenerated cellulose fiber. Because it is produced from naturally occurring polymers, it is neither a truly synthetic fiber nor a natural fiber; it is a semi-synthetic or artificial fiber. Rayon is known by the names viscose rayon and art silk in the textile industry...
. Osmium–iridium is used for compass
Compass
A compass is a navigational instrument that shows directions in a frame of reference that is stationary relative to the surface of the earth. The frame of reference defines the four cardinal directions – north, south, east, and west. Intermediate directions are also defined...
bearings and for balances.
Corrosion and heat resistance makes iridium an important alloying agent. Certain long-life aircraft engine parts are made of an iridium alloy and an iridium–titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
alloy is used for deep-water pipes because of its corrosion resistance. Iridium is also used as a hardening agent in platinum alloys. The Vickers hardness of pure platinum is 56 HV while platinum with 50% of iridium can reach over 500 HV.
Devices that must withstand extremely high temperatures are often made from iridium. For example, high-temperature crucible
Crucible
A crucible is a container used for metal, glass, and pigment production as well as a number of modern laboratory processes, which can withstand temperatures high enough to melt or otherwise alter its contents...
s made of iridium are used in the Czochralski process
Czochralski process
The Czochralski process is a method of crystal growth used to obtain single crystals of semiconductors , metals , salts, and synthetic gemstones...
to produce oxide single-crystals (such as sapphire
Sapphire
Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
s) for use in computer memory devices and in solid state lasers. The crystals, such as gadolinium gallium garnet
Gadolinium gallium garnet
Gadolinium Gallium Garnet is a synthetic crystalline material of the garnet group, with good mechanical, thermal, and optical properties. It is typically colorless. It has cubic lattice, density 7.08 g/cm³ and Mohs hardness is variously noted as 6.5 and 7.5...
and yttrium gallium garnet, are grown by melting pre-sintered charges of mixed oxides under oxidizing conditions at temperatures up to 2100 °C. Its resistance to arc erosion makes iridium alloys ideal for electrical contacts for spark plug
Spark plug
A spark plug is an electrical device that fits into the cylinder head of some internal combustion engines and ignites compressed fuels such as aerosol, gasoline, ethanol, and liquefied petroleum gas by means of an electric spark.Spark plugs have an insulated central electrode which is connected by...
s.
Iridium compounds are used as catalysts
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
in the Cativa process
Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc...
for carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
to produce acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. Iridium itself is used as a catalyst in a type of automobile engine introduced in 1996 called the direct-ignition engine.
The radioisotope iridium-192 is one of the two most important sources of energy for use in industrial γ-radiography for non-destructive testing of metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s. Additionally, 192Ir is used as a source of gamma radiation for the treatment of cancer using brachytherapy
Brachytherapy
Brachytherapy , also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment...
, a form of radiotherapy where a sealed radioactive source is placed inside or next to the area requiring treatment. Specific treatments include high dose rate prostate brachytherapy, bilary duct brachytherapy, and intracavitary cervix brachytherapy.
Scientific
An alloy of 90% platinum and 10% iridium was used in 1889 to construct the International Prototype MeterInternational Prototype Meter
The metre was originally defined as one ten-millionth of the distance between the North Pole and the equator at the longitude of Paris. Because of the difficulty of reproducing this measurement, a platinum bar nominally of that length was constructed in 1799 and housed in the Archives de la...
and kilogram
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
mass, kept by the International Bureau of Weights and Measures near Paris
Paris
Paris is the capital and largest city in France, situated on the river Seine, in northern France, at the heart of the Île-de-France region...
. The meter bar was replaced as the definition of the fundamental unit of length in 1960 by a line in the atomic spectrum of krypton,The definition of the meter was changed again in 1983. The meter is currently defined as the distance traveled by light in a vacuum during a time interval of of a second. but the kilogram prototype is still the international standard of mass.
Iridium has been used in the radioisotope thermoelectric generator
Radioisotope thermoelectric generator
A radioisotope thermoelectric generator is an electrical generator that obtains its power from radioactive decay. In such a device, the heat released by the decay of a suitable radioactive material is converted into electricity by the Seebeck effect using an array of thermocouples.RTGs can be...
s of unmanned spacecraft such as the Voyager
Voyager program
The Voyager program is a U.S program that launched two unmanned space missions, scientific probes Voyager 1 and Voyager 2. They were launched in 1977 to take advantage of a favorable planetary alignment of the late 1970s...
, Viking
Viking program
The Viking program consisted of a pair of American space probes sent to Mars, Viking 1 and Viking 2. Each spacecraft was composed of two main parts, an orbiter designed to photograph the surface of Mars from orbit, and a lander designed to study the planet from the surface...
, Pioneer
Pioneer program
The Pioneer program is a series of United States unmanned space missions that was designed for planetary exploration. There were a number of such missions in the program, but the most notable were Pioneer 10 and Pioneer 11, which explored the outer planets and left the solar system...
, Cassini
Cassini-Huygens
Cassini–Huygens is a joint NASA/ESA/ASI spacecraft mission studying the planet Saturn and its many natural satellites since 2004. Launched in 1997 after nearly two decades of gestation, it includes a Saturn orbiter and an atmospheric probe/lander for the moon Titan, although it has also returned...
, Galileo, and New Horizons
New Horizons
New Horizons is a NASA robotic spacecraft mission currently en route to the dwarf planet Pluto. It is expected to be the first spacecraft to fly by and study Pluto and its moons, Charon, Nix, Hydra and S/2011 P 1. Its estimated arrival date at the Pluto-Charon system is July 14th, 2015...
. Iridium was chosen to encapsulate the plutonium-238
Plutonium-238
-External links:**...
fuel in the generator because it can withstand the operating temperatures of up to 2000 °C and for its great strength.
Another use concerns X-ray optics, especially X-ray telescopes. The mirrors of the Chandra X-ray Observatory
Chandra X-ray Observatory
The Chandra X-ray Observatory is a satellite launched on STS-93 by NASA on July 23, 1999. It was named in honor of Indian-American physicist Subrahmanyan Chandrasekhar who is known for determining the maximum mass for white dwarfs. "Chandra" also means "moon" or "luminous" in Sanskrit.Chandra...
are coated with a layer of iridium 60 nm thick. Iridium proved to be the best choice for reflecting X-rays after nickel, gold, and platinum were also tested. The iridium layer, which had to be smooth to within a few atoms, was applied by depositing iridium vapor under high vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
on a base layer of chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
.
Iridium is used in particle physics
Particle physics
Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics...
for the production of antiproton
Antiproton
The antiproton is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived since any collision with a proton will cause both particles to be annihilated in a burst of energy....
s, a form of antimatter
Antimatter
In particle physics, antimatter is the extension of the concept of the antiparticle to matter, where antimatter is composed of antiparticles in the same way that normal matter is composed of particles...
. Antiprotons are made by shooting a high-intensity proton beam at a conversion target, which needs to be made from a very high density material. Although tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
may be used instead, iridium has the advantage of better stability under the shock wave
Shock wave
A shock wave is a type of propagating disturbance. Like an ordinary wave, it carries energy and can propagate through a medium or in some cases in the absence of a material medium, through a field such as the electromagnetic field...
s induced by the temperature rise due to the incident beam.
C-H bond activation
Carbon–hydrogen bond activation or C−H activation may be defined as a reaction that cleaves a carbon–hydrogen bond. Often the term is restricted to reactions involving organometallic complexes and proceeding by coordination of a hydrocarbon to the inner-sphere of metal, either via an...
(C–H activation) is an area of research on reactions that cleave carbon–hydrogen bonds, which were traditionally regarded as unreactive. The first reported successes at activating C–H bonds in saturated hydrocarbons, published in 1982, used organometallic iridium complexes that undergo an oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
with the hydrocarbon.
Iridium complexes are being investigated as catalysts for asymmetric hydrogenation. These catalysts have been used in the synthesis of natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s and able to hydrogenate certain difficult substrates, such as unfunctionalized alkenes, enantioselectively (generating only one of the two possible enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s).
Iridium forms a variety of complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
of fundamental interest in triplet harvesting.
Historical

Fountain pen
A fountain pen is a nib pen that, unlike its predecessor the dip pen, contains an internal reservoir of water-based liquid ink. The pen draws ink from the reservoir through a feed to the nib and deposits it on paper via a combination of gravity and capillary action...
nibs. The first major use of iridium was in 1834 in nibs mounted on gold. Since 1944, the famous Parker 51
Parker 51
The Parker 51, introduced in 1941, is a famous fountain pen. Parker’s period advertising called it “The World’s Most Wanted Pen,” and this assertion was true although a little deceptive; the U.S.A. entered World War II in December 1941, and the War Production Board placed severe restrictions on...
fountain pen was fitted with a nib tipped by a ruthenium and iridium alloy (with 3.8% iridium). The tip material in modern fountain pens is still conventionally called "iridium," although there is seldom any iridium in it; other metals such as tungsten have taken its place.
An iridium–platinum alloy was used for the touch hole
Touch hole
A touch hole is a small hole, through which the propellant charge of a cannon or muzzleloading gun is ignited. In small arms, the flash from a charge of priming held in the flash pan is enough to ignite the charge within...
s or vent pieces of cannon
Cannon
A cannon is any piece of artillery that uses gunpowder or other usually explosive-based propellents to launch a projectile. Cannon vary in caliber, range, mobility, rate of fire, angle of fire, and firepower; different forms of cannon combine and balance these attributes in varying degrees,...
. According to a report of the Paris Exhibition of 1867
Exposition Universelle (1867)
The Exposition Universelle of 1867 was a World Exposition held in Paris, France, in 1867.-Conception:In 1864, Emperor Napoleon III decreed that an international exposition should be held in Paris in 1867. A commission was appointed with Prince Jerome Napoleon as president, under whose direction...
, one of the pieces being exhibited by Johnson and Matthey "has been used in a Withworth gun for more than 3000 rounds, and scarcely shows signs of wear yet. Those who know the constant trouble and expense which are occasioned by the wearing of the vent-pieces of cannon when in active service, will appreciate this important adaptation".
The pigment iridium black, which consists of very finely divided iridium, is used for painting porcelain
Porcelain
Porcelain is a ceramic material made by heating raw materials, generally including clay in the form of kaolin, in a kiln to temperatures between and...
an intense black; it was said that "all other porcelain black colors appear grey by the side of it".
Precautions
Iridium in bulk metallic form is not biologically important or hazardous to health due to its lack of reactivity with tissues; there are only about 20 parts per trillion of iridium in human tissue. However, finely divided iridium powder can be hazardous to handle, as it is an irritant and may ignite in air.Very little is known about the toxicity of iridium compounds because they are used in very small amounts, but soluble salts, such as the iridium halides, could be hazardous due to elements other than iridium or due to iridium itself. However, most iridium compounds are insoluble, which makes absorption into the body difficult.
A radioisotope of iridium, , is dangerous like other radioactive isotopes. The only reported injuries related to iridium concern accidental exposure to radiation from used in brachytherapy
Brachytherapy
Brachytherapy , also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment...
. High-energy gamma radiation from can increase the risk of cancer. External exposure can cause burns, radiation poisoning
Radiation poisoning
Acute radiation syndrome also known as radiation poisoning, radiation sickness or radiation toxicity, is a constellation of health effects which occur within several months of exposure to high amounts of ionizing radiation...
, and death. Ingestion of 192Ir can burn the linings of the stomach and the intestines. 192Ir, 192mIr, and 194mIr tend to deposit in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, and can pose health hazards from both gamma and beta radiation.

