Chloralkali process
Encyclopedia
The chloralkali process is an industrial process for the electrolysis
of sodium chloride
solution (brine
). Depending on the method several products beside hydrogen
can be produced. If the products are separated, chlorine
and sodium hydroxide (caustic soda) are the products; by mixing, sodium hypochlorite or sodium chlorate
are produced, depending on the temperature. Higher temperatures are needed for the production of sodium chlorate instead of sodium hypochlorite. Industrial scale production began in 1892.
When using calcium chloride
or potassium chloride
, the products contain calcium or potassium instead of sodium.
The process has a high energy consumption, for example over 4 billion kWh per year in West Germany 1985, and produces equal (molar) amounts of chlorine and sodium hydroxide, which makes it necessary to find a use for the product for which there is less demand, usually the chlorine.
cell method produces chlorine-free sodium hydroxide, the use of several tonnes of mercury leads to serious environmental problems. In normal production cycle a few hundred pounds of mercury per year are emitted which accumulate in the environment. Additionally, the chlorine and sodium hydroxide produced via the mercury-cell chloralkali process are themselves contaminated with trace amounts of mercury. The membrane and diaphragm method use no mercury, but the sodium hydroxide contains chlorine which has to be removed.
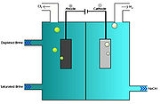
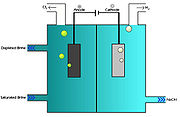 The most common chlorlakali process involves the electrolysis of aqueous sodium chloride
The most common chlorlakali process involves the electrolysis of aqueous sodium chloride
(a brine
) in a membrane cell.
Saturated brine is passed into the first chamber of the cell where the chloride
ions are oxidised
at the anode
to chlorine
:
At the cathode
, hydrogen
in the water is reduced
to hydrogen gas, releasing hydroxide
ions into the solution:
The non-permeable ion exchange membrane at the center of the cell allows the sodium
ions (Na+) to pass to the second chamber where they react with the hydroxide ions to produce caustic soda (NaOH). The overall reaction for the electrolysis of brine is thus:
A membrane cell is used to prevent the reaction between the chlorine and hydroxide ions. If this reaction were to occur the chlorine would be disproportionated
to form chloride and hypochlorite
ions:
Above about 60°C, chlorate
can be formed:
Because of the corrosive nature of chlorine production, the anode has to be made from a non-reactive metal such as titanium
, whereas the cathode can be made from Nickel
.
In the membrane cell, the anode and cathode are separated by an ion-permeable membrane. Saturated brine is fed to the compartment with the anode (the anolyte). A DC current is passed through the cell and the NaCl splits into its constituent components. The membrane passes Na+ ions to the cathode compartment (catholyte), where it forms sodium hydroxide in solution. The membrane allows only positive ions to pass through to prevent the chlorine from mixing with the sodium hydroxide. The chloride ions are oxidised to chlorine gas at the anode, which is collected, purified and stored. Hydrogen gas and Hydroxide ions are formed at the cathode.
. Brine is introduced into the anode compartment and flows into the cathode compartment. Similarly to the Membrane Cell, chloride ions are oxidized at the anode to produce chlorine, and at the cathode, water is split into caustic soda and hydrogen. The diaphragm prevents the reaction of the caustic soda with the chlorine. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50% and the salt removed. This is done using an evaporative process with about three tonnes of steam per tonne of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.
, a saturated brine solution floats on top of the cathode which is a thin layer of mercury. Chlorine is produced at the anode, and sodium is produced at the cathode where it forms a sodium-mercury amalgam with the mercury. The amalgam is continuously drawn out of the cell and reacted with water which decomposes the amalgam into sodium hydroxide and mercury. The mercury is recycled into the electrolytic cell. Mercury cells are being phased out due to concerns about mercury poisoning
from mercury cell pollution such as occurred in Canada (see Ontario Minamata disease
) and Japan (see Minamata disease
).
and The World Chlorine Council
.
can be made of a length of bent hose (a metal pipe should not be used) to connect the two beakers. Plug the ends with tissue or cloth. Put any electrode in the solution that you want to produce sodium hydroxide and hydrogen with. Put an electrode made from a carbon rod (or a pencil lead) into the solution that you want to produce the chlorine gas. If you want the hydrochloric and hypochlorous acid from the chlorine dissolution, put its electrode in the pure water solution. If you want the sodium hydroxide and hydrogen gas, put its electrode into the pure water solution. Connect the "any" electrode to the negative terminal of a 12 volt power supply.
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
of sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
solution (brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
). Depending on the method several products beside hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
can be produced. If the products are separated, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
and sodium hydroxide (caustic soda) are the products; by mixing, sodium hypochlorite or sodium chlorate
Sodium chlorate
Sodium chlorate is a chemical compound with the chemical formula . When pure, it is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 250 °C to release oxygen and leave sodium chloride...
are produced, depending on the temperature. Higher temperatures are needed for the production of sodium chlorate instead of sodium hypochlorite. Industrial scale production began in 1892.
When using calcium chloride
Calcium chloride
Calcium chloride, CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide, and is solid at room temperature. Common applications include brine for refrigeration plants, ice and dust control on roads, and desiccation...
or potassium chloride
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
, the products contain calcium or potassium instead of sodium.
The process has a high energy consumption, for example over 4 billion kWh per year in West Germany 1985, and produces equal (molar) amounts of chlorine and sodium hydroxide, which makes it necessary to find a use for the product for which there is less demand, usually the chlorine.
Procedures
There are three production methods in use. While the mercuryMercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
cell method produces chlorine-free sodium hydroxide, the use of several tonnes of mercury leads to serious environmental problems. In normal production cycle a few hundred pounds of mercury per year are emitted which accumulate in the environment. Additionally, the chlorine and sodium hydroxide produced via the mercury-cell chloralkali process are themselves contaminated with trace amounts of mercury. The membrane and diaphragm method use no mercury, but the sodium hydroxide contains chlorine which has to be removed.
Membrane cell

Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
(a brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
) in a membrane cell.
Saturated brine is passed into the first chamber of the cell where the chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
ions are oxidised
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
at the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
to chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
:
- 2Cl– → + 2e–ElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
At the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in the water is reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
to hydrogen gas, releasing hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions into the solution:
- 2 + 2e– → H2 + 2OH–
The non-permeable ion exchange membrane at the center of the cell allows the sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
ions (Na+) to pass to the second chamber where they react with the hydroxide ions to produce caustic soda (NaOH). The overall reaction for the electrolysis of brine is thus:
- 2NaCl + 2 → + + 2NaOH
A membrane cell is used to prevent the reaction between the chlorine and hydroxide ions. If this reaction were to occur the chlorine would be disproportionated
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
to form chloride and hypochlorite
Hypochlorite
The hypochlorite ion, also known as chlorate anion is ClO−. A hypochlorite compound is a chemical compound containing this group, with chlorine in oxidation state +1.Hypochlorites are the salts of hypochlorous acid...
ions:
- + 2OH– → Cl– + ClO– +
Above about 60°C, chlorate
Chlorate
The chlorate anion has the formula ClO. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a roman numeral in parentheses, e.g...
can be formed:
- 3 + 6OH– → 5Cl– + – + 3
Because of the corrosive nature of chlorine production, the anode has to be made from a non-reactive metal such as titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, whereas the cathode can be made from Nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
.
In the membrane cell, the anode and cathode are separated by an ion-permeable membrane. Saturated brine is fed to the compartment with the anode (the anolyte). A DC current is passed through the cell and the NaCl splits into its constituent components. The membrane passes Na+ ions to the cathode compartment (catholyte), where it forms sodium hydroxide in solution. The membrane allows only positive ions to pass through to prevent the chlorine from mixing with the sodium hydroxide. The chloride ions are oxidised to chlorine gas at the anode, which is collected, purified and stored. Hydrogen gas and Hydroxide ions are formed at the cathode.
Diaphragm cell
In the diaphragm cell process, there are two compartments separated by a permeable diaphragm, often made of asbestos fibersAsbestos
Asbestos is a set of six naturally occurring silicate minerals used commercially for their desirable physical properties. They all have in common their eponymous, asbestiform habit: long, thin fibrous crystals...
. Brine is introduced into the anode compartment and flows into the cathode compartment. Similarly to the Membrane Cell, chloride ions are oxidized at the anode to produce chlorine, and at the cathode, water is split into caustic soda and hydrogen. The diaphragm prevents the reaction of the caustic soda with the chlorine. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50% and the salt removed. This is done using an evaporative process with about three tonnes of steam per tonne of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.
Mercury cell
In the mercury-cell process, also known as the Castner-Kellner processCastner-Kellner process
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Karl Kellner in the 1890s....
, a saturated brine solution floats on top of the cathode which is a thin layer of mercury. Chlorine is produced at the anode, and sodium is produced at the cathode where it forms a sodium-mercury amalgam with the mercury. The amalgam is continuously drawn out of the cell and reacted with water which decomposes the amalgam into sodium hydroxide and mercury. The mercury is recycled into the electrolytic cell. Mercury cells are being phased out due to concerns about mercury poisoning
Mercury poisoning
Mercury poisoning is a disease caused by exposure to mercury or its compounds. Mercury is a heavy metal occurring in several forms, all of which can produce toxic effects in high enough doses...
from mercury cell pollution such as occurred in Canada (see Ontario Minamata disease
Ontario Minamata disease
Ontario Minamata disease is a neurological syndrome caused by severe mercury poisoning. It occurred in the Canadian province of Ontario in 1970 and severely affected two First Nation communities located in Northwestern Ontario following consumption of local fish that were contaminated with mercury...
) and Japan (see Minamata disease
Minamata disease
', sometimes referred to as , is a neurological syndrome caused by severe mercury poisoning. Symptoms include ataxia, numbness in the hands and feet, general muscle weakness, narrowing of the field of vision and damage to hearing and speech. In extreme cases, insanity, paralysis, coma, and death...
).
Manufacturer Associations
The interests of chloralkali product manufacturers are represented at regional, national and international levels by associations such as Euro ChlorEuro Chlor
Euro Chlor is an association of chloralkali process plant operators in Europe. It was originally founded in 1969 and is based in Brussels. Euro Chlor is a member association of the World Chlorine Council....
and The World Chlorine Council
World Chlorine Council
The World Chlorine Council is an international network of national and regional trade associations representing the chlorine and chlorinated products industries in over 27 countries. Members include chloralkali process associations such as Euro Chlor, Japan Soda Industry Association, Alkali...
.
Laboratory procedure
Electrolysis can be done with two beakers, one containing a brine and one containing pure water. A salt bridgeSalt bridge
A salt bridge, in chemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell , a type of electrochemical cell...
can be made of a length of bent hose (a metal pipe should not be used) to connect the two beakers. Plug the ends with tissue or cloth. Put any electrode in the solution that you want to produce sodium hydroxide and hydrogen with. Put an electrode made from a carbon rod (or a pencil lead) into the solution that you want to produce the chlorine gas. If you want the hydrochloric and hypochlorous acid from the chlorine dissolution, put its electrode in the pure water solution. If you want the sodium hydroxide and hydrogen gas, put its electrode into the pure water solution. Connect the "any" electrode to the negative terminal of a 12 volt power supply.

