
Osmium
Encyclopedia
Osmium is a chemical element
with the symbol Os and atomic number
76. Osmium is a hard, brittle, blue-gray or blue-black
transition metal
in the platinum family, and is the densest natural element. Osmium is twice as dense as lead
. The density of osmium is , slightly greater than that of iridium
, the second densest element. Osmium is found in nature as an alloy, mostly in platinum ores. Osmium is also used in alloy
s, with platinum
, iridium
and other platinum group metals. Those alloys are employed in fountain pen
tips, electrical contacts and in other applications where extreme durability and hardness are needed.
. Calculations of density from the X-ray diffraction data may produce the most reliable data for these elements, giving a value of for iridium versus for osmium. The high density of osmium is a consequence of the lanthanide contraction
.
Osmium is a hard but brittle metal
that remains lustrous even at high temperatures. It has a very low compressibility. Correspondingly, its bulk modulus
is extremely high, reported between and , which rivals that of diamond
. The hardness of osmium is moderately high at . Because of its hardness, brittleness, low vapor pressure
(the lowest of the platinum group metals), and very high melting point (the fourth highest of all elements), solid osmium is difficult to machine, form or work.
s ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element, and aside from osmium, is encountered only in xenon
, iron
and ruthenium
. The oxidation state −1 and −2 represented by the two reactive compounds and are used in the synthesis of osmium cluster compounds.
The most common compound exhibiting the +8 oxidation state is osmium tetroxide. This toxic compound is formed when powdered osmium is exposed to air, and is a very volatile, water-soluble, pale yellow, crystalline solid with a strong smell. Therefore, osmium powder has a characteristic smell of osmium tetroxide. Osmium tetroxide forms red osmates upon reaction with a base. With ammonia, it forms the nitrido-osmates . Osmium tetroxide boils at 130 °C
and is a powerful oxidizing agent. By contrast, osmium dioxide
(OsO2) is black, non-volatile, and much less reactive and toxic.
Only two osmium compounds have major applications: osmium tetroxide — for staining tissue in electron microscopy and the non-volatile osmates for organic oxidation reactions
.
Osmium heptafluoride (OsF7) and osmium pentafluoride (OsF5) are known, but osmium trifluoride (OsF3) has not been synthesized yet. The lower oxidation states are stabilized by the larger halogens. Therefore, the trichloride, tribromide, triiodide and even osmium diiodide are known. The oxidation state +1 is only known for the osmium iodide (OsI), whereas several carbonyl complexes of osmium, such as triosmium dodecacarbonyl
, represent the oxidation state 0.
In general, the lower oxidation states of osmium are stabilized by ligand
s that are good σ-donors (such as amines) and π-acceptors (heterocycle
s containing nitrogen
). The higher oxidation states are stabilized by strong σ- and π-donors, such as and .
s, six of which are stable: , , , , , and (most abundant) . undergoes alpha decay
with such long half-life
((2.0±1.1) years) that for practical purposes it can be considered stable. Alpha decay is predicted for all 7 naturally occurring isotopes, but due to very long half-lives, it was observed only for . It is predicted that and can undergo double beta decay
but this radioactivity has not been observed yet.
is the daughter of (half-life ) and is used extensively in dating terrestrial as well as meteoric
rock
s (see rhenium-osmium dating
). It has also been used to measure the intensity of continental weathering over geologic time and to fix minimum ages for stabilization of the mantle roots of continental craton
s. This decay is a reason why rhenium-rich minerals are abnormally rich in . However, the most notable application of Os in dating has been in conjunction with iridium, to analyze the layer of shocked quartz
along the K-T boundary
that marks the extinction of the dinosaur
s 65 million years ago.
osme (ὀσμή) meaning "smell") was discovered in 1803 by Smithson Tennant
and William Hyde Wollaston
in London
, England
. The discovery of osmium is intertwined with that of platinum and the other metals of the platinum group
. Platinum reached Europe as platina ("small silver"), first encountered in the late 17th century in silver mines around the Chocó Department
, in Colombia
. The discovery that this metal was not an alloy, but a distinct new element, was published in 1748.
Chemists who studied platinum dissolved it in aqua regia
(a mixture of hydrochloric
and nitric acid
s) to create soluble salts. They always observed a small amount of a dark, insoluble residue. Joseph Louis Proust thought that the residue was graphite
. Victor Collet-Descotils, Antoine François, comte de Fourcroy
, and Louis Nicolas Vauquelin
also observed the black residue in 1803, but did not obtain enough material for further experiments.
In 1803, Smithson Tennant
analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin treated the powder alternately with alkali and acids and obtained a volatile new oxide, which he believed to be of this new metal—which he named ptene, from the Greek word (ptènos) for winged. However, Tennant, who had the advantage of a much larger amount of residue, continued his research and identified two previously undiscovered elements in the black residue, iridium and osmium. He obtained a yellow solution (probably of cis–[ Os(OH)2O4] 2−) by reactions with sodium hydroxide at red heat. After acidification he was able to distill the formed OsO4. He named osmium after Greek
osme meaning "a smell", because of the smell of the volatile osmium tetroxide. Discovery of the new elements was documented in a letter to the Royal Society
on June 21, 1804.
Uranium and osmium were early successful catalysts in the Haber process
, the nitrogen fixation
reaction of nitrogen
and hydrogen
to produce ammonia
, giving enough yield to make the process economically successful. However, in 1908 cheaper catalysts based on iron and iron oxides were introduced for the first pilot plants.
Nowadays, osmium is primarily obtained from the processing of platinum
and nickel
ores.
stable element in the Earth's crust
with an average mass fraction of 0.05 ppb in the continental crust
.
Osmium is found in nature as an uncombined element or in natural alloy
s; especially the iridium–osmium alloys, osmiridium
(osmium rich), and iridiosmium (iridium rich). In the nickel
and copper
deposits, the platinum group metals occur as sulfide
s (i.e., (Pt,Pd)S)), tellurides
(e.g., PtBiTe), antimonide
s (e.g., PdSb), and arsenide
s (e.g., PtAs2); in all these compounds platinum is exchanged by a small amount of iridium and osmium. As with all of the platinum group metals, osmium can be found naturally in alloys with nickel or copper
.
Within the Earth's crust, osmium, like iridium, is found at highest concentrations in three types of geologic structure: igneous deposits (crustal intrusions from below), impact craters, and deposits reworked from one of the former structures. The largest known primary reserves are in the Bushveld igneous complex
in South Africa
, though the large copper–nickel deposits near Norilsk in Russia
, and the Sudbury Basin
in Canada
are also significant sources of osmium. Smaller reserves can be found in the United States. The alluvial deposits used by pre-Columbian
people in the Chocó Department
, Colombia
are still a source for platinum group metals. The second large alluvial deposit was found in the Ural Mountains
, Russia, which is still mined.
and copper
mining and processing. During electrorefining of copper and nickel, noble metals such as silver, gold and the platinum group metals, together with non-metallic elements such as selenium
and tellurium settle to the bottom of the cell as anode mud, which forms the starting material for their extraction. In order to separate the metals, they must first be brought into solution. Several methods are available depending on the separation process and the composition of the mixture; two representative methods are fusion with sodium peroxide
followed by dissolution in aqua regia
, and dissolution in a mixture of chlorine
with hydrochloric acid
. Osmium, ruthenium, rhodium and iridium can be separated from platinum, gold and base metals by their insolubility in aqua regia, leaving a solid residue. Rhodium can be separated from the residue by treatment with molten sodium bisulfate
. The insoluble residue, containing Ru, Os and Ir, is treated with sodium oxide
, in which Ir is insoluble, producing water-soluble Ru and Os salts. After oxidation to the volatile oxides, is separated from by precipitation of (NH4)3RuCl6 with ammonium chloride.
After it is dissolved, osmium is separated from the other platinum group metals by distillation or extraction with organic solvents of the volatile osmium tetroxide. The first method is similar to the procedure used by Tennant and Wollaston. Both methods are suitable for industrial scale production. In either case, the product is reduced using hydrogen, yielding the metal as a powder or sponge that can be treated using powder metallurgy
techniques.
Neither the producers nor the United States Geological Survey published any production amounts for osmium. Estimations of the United States consumption date published from 1971, which gives a consumption in the United States of 2000 troy ounce
s (62 kg), would suggest that the production is still less than 1 ton per year.
 Because of the volatility and extreme toxicity of its oxide, osmium is rarely used in its pure state, and is instead often alloyed with other metals. Those alloys are utilized in high-wear applications. Osmium alloys such as osmiridium
Because of the volatility and extreme toxicity of its oxide, osmium is rarely used in its pure state, and is instead often alloyed with other metals. Those alloys are utilized in high-wear applications. Osmium alloys such as osmiridium
are very hard and, along with other platinum group metals, are used in the tips of fountain pen
s, instrument pivots, and electrical contacts, as they can resist wear from frequent operation. The stylus (needle) in early phonograph
designs was also made of osmium, especially for 78-rpm records, until sapphire
and synthetic diamond
replaced the metal in later designs for 45-rpm and 33-rpm long-playing records.
 Osmium tetroxide has been used in fingerprint
Osmium tetroxide has been used in fingerprint
detection and in staining fat
ty tissue for optical and electron microscopy. As a strong oxidant, it cross-links lipids mainly by reacting with unsaturated carbon-carbon bonds, and thereby both fixes biological membranes in place in tissue samples and simultaneously stains them. Because osmium atoms are extremely electron dense, osmium staining greatly enhances image contrast in transmission electron microscopy
(TEM) studies of biological materials. Those carbon materials have otherwise very weak TEM contrast (see image). Another osmium compound, osmium ferricyanide (OsFeCN), exhibits similar fixing and staining action.
An alloy of 90% platinum and 10% osmium is used in surgical implant
s such as pacemaker
s and replacement of pulmonary valves.
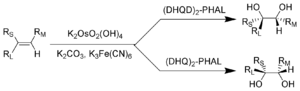
The tetroxide and a related compound, potassium osmate, are important oxidants for chemical synthesis, despite being very poisonous. For the Sharpless asymmetric dihydroxylation
, which uses osmate for the conversion of a double bond
into a vicinal
diol
, Karl Barry Sharpless won the Nobel Prize in Chemistry
in 2001.
 In 1898 an Austrian chemist, Auer von Welsbach
In 1898 an Austrian chemist, Auer von Welsbach
, developed the Oslamp with a filament made of osmium, which he introduced commercially in 1902. After only a few years, osmium was replaced by the more stable metal tungsten
(also known as wolfram). Tungsten has the highest melting point of any metal, and using it in light bulbs increases the luminous efficacy and life of incandescent lamps.
The light bulb manufacturer OSRAM
(founded in 1906 when three German companies, Auer-Gesellschaft, AEG and Siemens & Halske, combined their lamp production facilities) derived its name from the elements of OSmium and wolfRAM.
Like palladium, powdered osmium effectively absorbs hydrogen atoms. This could make osmium a potential candidate for a metal hydride battery electrode. However, osmium is expensive and would react with potassium hydroxide, the most common battery electrolyte.
Osmium has high reflectivity in the ultraviolet
range of the electromagnetic spectrum
; for example, at 600 Å
osmium has a reflectivity twice that of gold. This high reflectivity is desirable in space-based UV spectrometers
which have reduced mirror sizes due to space limitations. Osmium-coated mirrors were flown in several space missions aboard the Space Shuttle
, but it soon became clear that the oxygen radicals in the low earth orbit
are abundant enough to significantly deteriorate the osmium layer.
.
Osmium reacts with oxygen at room temperature forming volatile osmium tetroxide. Some osmium compounds are also converted to the tetroxide if oxygen is present.
This makes osmium tetroxide the main source of contact with the environment.
Osmium tetroxide is highly volatile and penetrates skin readily, and is very toxic by inhalation, ingestion, and skin contact. Airborne low concentrations of osmium tetroxide vapor can cause lung
congestion and skin
or eye
damage, and should therefore be used in a fume hood. Osmium tetroxide is rapidly reduced to relatively inert compounds by polyunsaturated vegetable oils, such as corn oil
.
and by grams. Its price at 2010 is about $400 per Troy ounce (or about $13 per gram), depending on the quantity and its supplier.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol Os and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
76. Osmium is a hard, brittle, blue-gray or blue-black
transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
in the platinum family, and is the densest natural element. Osmium is twice as dense as lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
. The density of osmium is , slightly greater than that of iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
, the second densest element. Osmium is found in nature as an alloy, mostly in platinum ores. Osmium is also used in alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s, with platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
and other platinum group metals. Those alloys are employed in fountain pen
Fountain pen
A fountain pen is a nib pen that, unlike its predecessor the dip pen, contains an internal reservoir of water-based liquid ink. The pen draws ink from the reservoir through a feed to the nib and deposits it on paper via a combination of gravity and capillary action...
tips, electrical contacts and in other applications where extreme durability and hardness are needed.
Physical properties
Osmium has a blue-gray tint and is the densest stable element, slightly denser than iridiumIridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
. Calculations of density from the X-ray diffraction data may produce the most reliable data for these elements, giving a value of for iridium versus for osmium. The high density of osmium is a consequence of the lanthanide contraction
Lanthanide contraction
Lanthanide contraction is a term used in chemistry to describe the decrease in ionic radii of the elements in the lanthanide series from atomic number 58, Cerium to 71, Lutetium, which results in smaller than otherwise expected ionic radii for the subsequent elements starting with 72, Hafnium...
.
Osmium is a hard but brittle metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
that remains lustrous even at high temperatures. It has a very low compressibility. Correspondingly, its bulk modulus
Bulk modulus
The bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e...
is extremely high, reported between and , which rivals that of diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
. The hardness of osmium is moderately high at . Because of its hardness, brittleness, low vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
(the lowest of the platinum group metals), and very high melting point (the fourth highest of all elements), solid osmium is difficult to machine, form or work.
Chemical properties
Osmium forms compounds with the oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element, and aside from osmium, is encountered only in xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
, iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
and ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
. The oxidation state −1 and −2 represented by the two reactive compounds and are used in the synthesis of osmium cluster compounds.
The most common compound exhibiting the +8 oxidation state is osmium tetroxide. This toxic compound is formed when powdered osmium is exposed to air, and is a very volatile, water-soluble, pale yellow, crystalline solid with a strong smell. Therefore, osmium powder has a characteristic smell of osmium tetroxide. Osmium tetroxide forms red osmates upon reaction with a base. With ammonia, it forms the nitrido-osmates . Osmium tetroxide boils at 130 °C
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
and is a powerful oxidizing agent. By contrast, osmium dioxide
Osmium dioxide
Osmium dioxide is an inorganic compound with the formula OsO2. It exists as brown to black crystalline powder, but single crystals are golden and exhibit metallic conductivity. The compound crystallizes in the rutile structural motif, i.e...
(OsO2) is black, non-volatile, and much less reactive and toxic.
Only two osmium compounds have major applications: osmium tetroxide — for staining tissue in electron microscopy and the non-volatile osmates for organic oxidation reactions
Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
.
Osmium heptafluoride (OsF7) and osmium pentafluoride (OsF5) are known, but osmium trifluoride (OsF3) has not been synthesized yet. The lower oxidation states are stabilized by the larger halogens. Therefore, the trichloride, tribromide, triiodide and even osmium diiodide are known. The oxidation state +1 is only known for the osmium iodide (OsI), whereas several carbonyl complexes of osmium, such as triosmium dodecacarbonyl
Triosmium dodecacarbonyl
Triosmium dodecacarbonyl is a chemical compound with the formula Os312. This yellow-colored metal carbonyl cluster is an important precursor to organo-osmium compounds...
, represent the oxidation state 0.
In general, the lower oxidation states of osmium are stabilized by ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s that are good σ-donors (such as amines) and π-acceptors (heterocycle
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
s containing nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
). The higher oxidation states are stabilized by strong σ- and π-donors, such as and .
Isotopes
Osmium has seven naturally occurring isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, six of which are stable: , , , , , and (most abundant) . undergoes alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
with such long half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
((2.0±1.1) years) that for practical purposes it can be considered stable. Alpha decay is predicted for all 7 naturally occurring isotopes, but due to very long half-lives, it was observed only for . It is predicted that and can undergo double beta decay
Double beta decay
Double beta decay is a radioactive decay process where a nucleus releases two beta rays as a single process.In double-beta decay, two neutrons in the nucleus are converted to protons, and two electrons and two electron antineutrinos are emitted...
but this radioactivity has not been observed yet.
is the daughter of (half-life ) and is used extensively in dating terrestrial as well as meteoric
Meteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
rock
Rock (geology)
In geology, rock or stone is a naturally occurring solid aggregate of minerals and/or mineraloids.The Earth's outer solid layer, the lithosphere, is made of rock. In general rocks are of three types, namely, igneous, sedimentary, and metamorphic...
s (see rhenium-osmium dating
Rhenium-osmium dating
Rhenium-Osmium dating is a form of radiometric dating based on the beta decay of the isotope 187Re to 187Os. This normally occurs with a half-life of 41.6 × 109 y, but studies using fully ionised 187Re atoms have found that this can decrease to only 33 y...
). It has also been used to measure the intensity of continental weathering over geologic time and to fix minimum ages for stabilization of the mantle roots of continental craton
Craton
A craton is an old and stable part of the continental lithosphere. Having often survived cycles of merging and rifting of continents, cratons are generally found in the interiors of tectonic plates. They are characteristically composed of ancient crystalline basement rock, which may be covered by...
s. This decay is a reason why rhenium-rich minerals are abnormally rich in . However, the most notable application of Os in dating has been in conjunction with iridium, to analyze the layer of shocked quartz
Shocked quartz
Shocked quartz is a form of quartz that has a microscopic structure that is different from normal quartz. Under intense pressure , the crystalline structure of quartz will be deformed along planes inside the crystal...
along the K-T boundary
Cretaceous-Tertiary extinction event
The Cretaceous–Paleogene extinction event, formerly named and still commonly referred to as the Cretaceous-Tertiary extinction event, occurred approximately 65.5 million years ago at the end of the Maastrichtian age of the Cretaceous period. It was a large-scale mass extinction of animal and plant...
that marks the extinction of the dinosaur
Dinosaur
Dinosaurs are a diverse group of animals of the clade and superorder Dinosauria. They were the dominant terrestrial vertebrates for over 160 million years, from the late Triassic period until the end of the Cretaceous , when the Cretaceous–Paleogene extinction event led to the extinction of...
s 65 million years ago.
History
Osmium (from GreekGreek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
osme (ὀσμή) meaning "smell") was discovered in 1803 by Smithson Tennant
Smithson Tennant
Smithson Tennant FRS was an English chemist.Tennant is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal. The mineral tennantite is...
and William Hyde Wollaston
William Hyde Wollaston
William Hyde Wollaston FRS was an English chemist and physicist who is famous for discovering two chemical elements and for developing a way to process platinum ore.-Biography:...
in London
London
London is the capital city of :England and the :United Kingdom, the largest metropolitan area in the United Kingdom, and the largest urban zone in the European Union by most measures. Located on the River Thames, London has been a major settlement for two millennia, its history going back to its...
, England
England
England is a country that is part of the United Kingdom. It shares land borders with Scotland to the north and Wales to the west; the Irish Sea is to the north west, the Celtic Sea to the south west, with the North Sea to the east and the English Channel to the south separating it from continental...
. The discovery of osmium is intertwined with that of platinum and the other metals of the platinum group
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
. Platinum reached Europe as platina ("small silver"), first encountered in the late 17th century in silver mines around the Chocó Department
Chocó Department
Chocó is a department of Colombia known for its large Afro-Colombian population. It is in the west of the country, and is the only Colombian department to have coastlines on both the Pacific Ocean and the Atlantic Ocean. It also has all of Colombia's border with Panama. Its capital is...
, in Colombia
Colombia
Colombia, officially the Republic of Colombia , is a unitary constitutional republic comprising thirty-two departments. The country is located in northwestern South America, bordered to the east by Venezuela and Brazil; to the south by Ecuador and Peru; to the north by the Caribbean Sea; to the...
. The discovery that this metal was not an alloy, but a distinct new element, was published in 1748.
Chemists who studied platinum dissolved it in aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
(a mixture of hydrochloric
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
s) to create soluble salts. They always observed a small amount of a dark, insoluble residue. Joseph Louis Proust thought that the residue was graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. Victor Collet-Descotils, Antoine François, comte de Fourcroy
Antoine François, comte de Fourcroy
Antoine François, comte de Fourcroy was a French chemist and a contemporary of Antoine Lavoisier. Fourcroy collaborated with Lavoisier, Guyton de Morveau, and Claude Berthollet on the Méthode de nomenclature chimique, a work that helped standardize chemical nomenclature.-Life and work:Fourcroy...
, and Louis Nicolas Vauquelin
Louis Nicolas Vauquelin
Nicolas Louis Vauquelin , was a French pharmacist and chemist.-Early life:Vauquelin was born at Saint-André-d'Hébertot in Normandy, France. His first acquaintance with chemistry was gained as laboratory assistant to an apothecary in Rouen , and after various vicissitudes he obtained an introduction...
also observed the black residue in 1803, but did not obtain enough material for further experiments.
In 1803, Smithson Tennant
Smithson Tennant
Smithson Tennant FRS was an English chemist.Tennant is best known for his discovery of the elements iridium and osmium, which he found in the residues from the solution of platinum ores in 1803. He also contributed to the proof of the identity of diamond and charcoal. The mineral tennantite is...
analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin treated the powder alternately with alkali and acids and obtained a volatile new oxide, which he believed to be of this new metal—which he named ptene, from the Greek word (ptènos) for winged. However, Tennant, who had the advantage of a much larger amount of residue, continued his research and identified two previously undiscovered elements in the black residue, iridium and osmium. He obtained a yellow solution (probably of cis–
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
osme meaning "a smell", because of the smell of the volatile osmium tetroxide. Discovery of the new elements was documented in a letter to the Royal Society
Royal Society
The Royal Society of London for Improving Natural Knowledge, known simply as the Royal Society, is a learned society for science, and is possibly the oldest such society in existence. Founded in November 1660, it was granted a Royal Charter by King Charles II as the "Royal Society of London"...
on June 21, 1804.
Uranium and osmium were early successful catalysts in the Haber process
Haber process
The Haber process, also called the Haber–Bosch process, is the nitrogen fixation reaction of nitrogen gas and hydrogen gas, over an enriched iron or ruthenium catalyst, which is used to industrially produce ammonia....
, the nitrogen fixation
Nitrogen fixation
Nitrogen fixation is the natural process, either biological or abiotic, by which nitrogen in the atmosphere is converted into ammonia . This process is essential for life because fixed nitrogen is required to biosynthesize the basic building blocks of life, e.g., nucleotides for DNA and RNA and...
reaction of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
to produce ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, giving enough yield to make the process economically successful. However, in 1908 cheaper catalysts based on iron and iron oxides were introduced for the first pilot plants.
Nowadays, osmium is primarily obtained from the processing of platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
ores.
Occurrence
Osmium is the least abundantAbundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
stable element in the Earth's crust
Crust (geology)
In geology, the crust is the outermost solid shell of a rocky planet or natural satellite, which is chemically distinct from the underlying mantle...
with an average mass fraction of 0.05 ppb in the continental crust
Continental crust
The continental crust is the layer of igneous, sedimentary, and metamorphic rocks which form the continents and the areas of shallow seabed close to their shores, known as continental shelves. This layer is sometimes called sial due to more felsic, or granitic, bulk composition, which lies in...
.
Osmium is found in nature as an uncombined element or in natural alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s; especially the iridium–osmium alloys, osmiridium
Osmiridium
Osmiridium, are names given to natural alloys of osmium and iridium, with traces of other platinum group metals. Osmiridium has been defined as containing a higher proportion of iridium, while iridosmine contains more osmium...
(osmium rich), and iridiosmium (iridium rich). In the nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
deposits, the platinum group metals occur as sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s (i.e., (Pt,Pd)S)), tellurides
Telluride (chemistry)
The telluride ion is Te2−. It is the final stable member of the series of dianions O2−, S2−, and Se2− ....
(e.g., PtBiTe), antimonide
Antimonide
Antimonides are compounds of antimony with more electropositive elements. The antimonide ion is Sb3−.Many of them are flammable or decomposed by oxygen when heated since the antimonide ion is a reducing agent....
s (e.g., PdSb), and arsenide
Arsenide
Arsenide is an arsenic anion with the charge −3. The trianion is formed by the reduction of arsenic by three electrons. For example heating arsenic powder with excess sodium gives sodium arsenide . The anions have no existence in solution since they are extremely basic...
s (e.g., PtAs2); in all these compounds platinum is exchanged by a small amount of iridium and osmium. As with all of the platinum group metals, osmium can be found naturally in alloys with nickel or copper
Native copper
Copper, as native copper, is one of the few metallic elements to occur in uncombined form as a natural mineral, although most commonly occurs in oxidized states and mixed with other elements...
.
Within the Earth's crust, osmium, like iridium, is found at highest concentrations in three types of geologic structure: igneous deposits (crustal intrusions from below), impact craters, and deposits reworked from one of the former structures. The largest known primary reserves are in the Bushveld igneous complex
Bushveld igneous complex
The Bushveld Igneous Complex is a large layered igneous intrusion within the Earth's crust which has been tilted and eroded and now outcrops around what appears to be the edge of a great geological basin, the Transvaal Basin. Located in South Africa, the BIC contains some of the richest ore...
in South Africa
South Africa
The Republic of South Africa is a country in southern Africa. Located at the southern tip of Africa, it is divided into nine provinces, with of coastline on the Atlantic and Indian oceans...
, though the large copper–nickel deposits near Norilsk in Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
, and the Sudbury Basin
Sudbury Basin
The Sudbury Basin, also known as Sudbury Structure or the Sudbury Nickel Irruptive, is a major geologic structure in Ontario, Canada. It is the second-largest known impact crater or astrobleme on Earth, as well as one of the oldest....
in Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
are also significant sources of osmium. Smaller reserves can be found in the United States. The alluvial deposits used by pre-Columbian
Pre-Columbian
The pre-Columbian era incorporates all period subdivisions in the history and prehistory of the Americas before the appearance of significant European influences on the American continents, spanning the time of the original settlement in the Upper Paleolithic period to European colonization during...
people in the Chocó Department
Chocó Department
Chocó is a department of Colombia known for its large Afro-Colombian population. It is in the west of the country, and is the only Colombian department to have coastlines on both the Pacific Ocean and the Atlantic Ocean. It also has all of Colombia's border with Panama. Its capital is...
, Colombia
Colombia
Colombia, officially the Republic of Colombia , is a unitary constitutional republic comprising thirty-two departments. The country is located in northwestern South America, bordered to the east by Venezuela and Brazil; to the south by Ecuador and Peru; to the north by the Caribbean Sea; to the...
are still a source for platinum group metals. The second large alluvial deposit was found in the Ural Mountains
Ural Mountains
The Ural Mountains , or simply the Urals, are a mountain range that runs approximately from north to south through western Russia, from the coast of the Arctic Ocean to the Ural River and northwestern Kazakhstan. Their eastern side is usually considered the natural boundary between Europe and Asia...
, Russia, which is still mined.
Production
Osmium is obtained commercially as a by-product from nickelNickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
mining and processing. During electrorefining of copper and nickel, noble metals such as silver, gold and the platinum group metals, together with non-metallic elements such as selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
and tellurium settle to the bottom of the cell as anode mud, which forms the starting material for their extraction. In order to separate the metals, they must first be brought into solution. Several methods are available depending on the separation process and the composition of the mixture; two representative methods are fusion with sodium peroxide
Sodium peroxide
Sodium peroxide is the inorganic compound with the formula Na2O2. This solid is the product when sodium is burned with oxygen. It is a strong base and a potent oxidizing agent. It exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and...
followed by dissolution in aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
, and dissolution in a mixture of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
with hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. Osmium, ruthenium, rhodium and iridium can be separated from platinum, gold and base metals by their insolubility in aqua regia, leaving a solid residue. Rhodium can be separated from the residue by treatment with molten sodium bisulfate
Sodium bisulfate
Sodium bisulfate, also known as sodium hydrogen sulfate , is an acid salt. It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of Sodium bisulfate, also known as sodium...
. The insoluble residue, containing Ru, Os and Ir, is treated with sodium oxide
Sodium oxide
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses, though not in a raw form. Treatment with water affords sodium hydroxide....
, in which Ir is insoluble, producing water-soluble Ru and Os salts. After oxidation to the volatile oxides, is separated from by precipitation of (NH4)3RuCl6 with ammonium chloride.
After it is dissolved, osmium is separated from the other platinum group metals by distillation or extraction with organic solvents of the volatile osmium tetroxide. The first method is similar to the procedure used by Tennant and Wollaston. Both methods are suitable for industrial scale production. In either case, the product is reduced using hydrogen, yielding the metal as a powder or sponge that can be treated using powder metallurgy
Powder metallurgy
Powder metallurgy is the process of blending fine powdered materials, pressing them into a desired shape , and then heating the compressed material in a controlled atmosphere to bond the material . The powder metallurgy process generally consists of four basic steps: powder manufacture, powder...
techniques.
Neither the producers nor the United States Geological Survey published any production amounts for osmium. Estimations of the United States consumption date published from 1971, which gives a consumption in the United States of 2000 troy ounce
Troy ounce
The troy ounce is a unit of imperial measure. In the present day it is most commonly used to gauge the weight of precious metals. One troy ounce is nowadays defined as exactly 0.0311034768 kg = 31.1034768 g. There are approximately 32.1507466 troy oz in 1 kg...
s (62 kg), would suggest that the production is still less than 1 ton per year.
Applications

Osmiridium
Osmiridium, are names given to natural alloys of osmium and iridium, with traces of other platinum group metals. Osmiridium has been defined as containing a higher proportion of iridium, while iridosmine contains more osmium...
are very hard and, along with other platinum group metals, are used in the tips of fountain pen
Fountain pen
A fountain pen is a nib pen that, unlike its predecessor the dip pen, contains an internal reservoir of water-based liquid ink. The pen draws ink from the reservoir through a feed to the nib and deposits it on paper via a combination of gravity and capillary action...
s, instrument pivots, and electrical contacts, as they can resist wear from frequent operation. The stylus (needle) in early phonograph
Phonograph
The phonograph record player, or gramophone is a device introduced in 1877 that has had continued common use for reproducing sound recordings, although when first developed, the phonograph was used to both record and reproduce sounds...
designs was also made of osmium, especially for 78-rpm records, until sapphire
Sapphire
Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
and synthetic diamond
Synthetic diamond
Synthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
replaced the metal in later designs for 45-rpm and 33-rpm long-playing records.

Fingerprint
A fingerprint in its narrow sense is an impression left by the friction ridges of a human finger. In a wider use of the term, fingerprints are the traces of an impression from the friction ridges of any part of a human hand. A print from the foot can also leave an impression of friction ridges...
detection and in staining fat
Fat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
ty tissue for optical and electron microscopy. As a strong oxidant, it cross-links lipids mainly by reacting with unsaturated carbon-carbon bonds, and thereby both fixes biological membranes in place in tissue samples and simultaneously stains them. Because osmium atoms are extremely electron dense, osmium staining greatly enhances image contrast in transmission electron microscopy
Transmission electron microscopy
Transmission electron microscopy is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through...
(TEM) studies of biological materials. Those carbon materials have otherwise very weak TEM contrast (see image). Another osmium compound, osmium ferricyanide (OsFeCN), exhibits similar fixing and staining action.
An alloy of 90% platinum and 10% osmium is used in surgical implant
Implant (medicine)
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue...
s such as pacemaker
Artificial pacemaker
A pacemaker is a medical device that uses electrical impulses, delivered by electrodes contacting the heart muscles, to regulate the beating of the heart...
s and replacement of pulmonary valves.
The tetroxide and a related compound, potassium osmate, are important oxidants for chemical synthesis, despite being very poisonous. For the Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
, which uses osmate for the conversion of a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
into a vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
, Karl Barry Sharpless won the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 2001.

Carl Auer von Welsbach
Carl Auer Freiherr von Welsbach was an Austrian scientist and inventor who had a talent for not only discovering advances, but turning them into commercially successful products...
, developed the Oslamp with a filament made of osmium, which he introduced commercially in 1902. After only a few years, osmium was replaced by the more stable metal tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
(also known as wolfram). Tungsten has the highest melting point of any metal, and using it in light bulbs increases the luminous efficacy and life of incandescent lamps.
The light bulb manufacturer OSRAM
Osram
Osram, founded 1919, is part of the industry sector of Siemens AG and one of the two leading lighting manufacturers in the world. The name is derived from osmium and Wolfram , as both these elements were commonly used for lighting filaments at the time the company was founded...
(founded in 1906 when three German companies, Auer-Gesellschaft, AEG and Siemens & Halske, combined their lamp production facilities) derived its name from the elements of OSmium and wolfRAM.
Like palladium, powdered osmium effectively absorbs hydrogen atoms. This could make osmium a potential candidate for a metal hydride battery electrode. However, osmium is expensive and would react with potassium hydroxide, the most common battery electrolyte.
Osmium has high reflectivity in the ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
range of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
; for example, at 600 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
osmium has a reflectivity twice that of gold. This high reflectivity is desirable in space-based UV spectrometers
Ultraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges...
which have reduced mirror sizes due to space limitations. Osmium-coated mirrors were flown in several space missions aboard the Space Shuttle
Space Shuttle
The Space Shuttle was a manned orbital rocket and spacecraft system operated by NASA on 135 missions from 1981 to 2011. The system combined rocket launch, orbital spacecraft, and re-entry spaceplane with modular add-ons...
, but it soon became clear that the oxygen radicals in the low earth orbit
Low Earth orbit
A low Earth orbit is generally defined as an orbit within the locus extending from the Earth’s surface up to an altitude of 2,000 km...
are abundant enough to significantly deteriorate the osmium layer.
Precautions
Finely divided metallic osmium is pyrophoricPyrophoricity
A pyrophoric substance is a substance that will ignite spontaneously in air. Examples are iron sulfide and many reactive metals including uranium, when powdered or sliced thin. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air...
.
Osmium reacts with oxygen at room temperature forming volatile osmium tetroxide. Some osmium compounds are also converted to the tetroxide if oxygen is present.
This makes osmium tetroxide the main source of contact with the environment.
Osmium tetroxide is highly volatile and penetrates skin readily, and is very toxic by inhalation, ingestion, and skin contact. Airborne low concentrations of osmium tetroxide vapor can cause lung
Lung
The lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
congestion and skin
Human skin
The human skin is the outer covering of the body. In humans, it is the largest organ of the integumentary system. The skin has multiple layers of ectodermal tissue and guards the underlying muscles, bones, ligaments and internal organs. Human skin is similar to that of most other mammals,...
or eye
Human eye
The human eye is an organ which reacts to light for several purposes. As a conscious sense organ, the eye allows vision. Rod and cone cells in the retina allow conscious light perception and vision including color differentiation and the perception of depth...
damage, and should therefore be used in a fume hood. Osmium tetroxide is rapidly reduced to relatively inert compounds by polyunsaturated vegetable oils, such as corn oil
Corn oil
Corn oil is oil extracted from the germ of corn . Its main use is in cooking, where its high smoke point makes refined corn oil a valuable frying oil. It is also a key ingredient in some margarines. Corn oil is generally less expensive than most other types of vegetable oils. One bushel of corn...
.
Price
Osmium is usually sold as a 99% pure powder. Like other precious metals, it is measured by troy weightTroy weight
Troy weight is a system of units of mass customarily used for precious metals, gemstones, and black powder.There are 12 troy ounces per troy pound, rather than the 16 ounces per pound found in the more common avoirdupois system. The troy ounce is 480 grains, compared with the avoirdupois ounce,...
and by grams. Its price at 2010 is about $400 per Troy ounce (or about $13 per gram), depending on the quantity and its supplier.
External links
- WebElements.com: Osmium
- Chemistry in its element podcast (MP3) from the Royal Society of ChemistryRoyal Society of ChemistryThe Royal Society of Chemistry is a learned society in the United Kingdom with the goal of "advancing the chemical sciences." It was formed in 1980 from the merger of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society and the Society for Analytical Chemistry with a new...
's Chemistry WorldChemistry WorldChemistry World is a monthly chemistry news magazine published by the Royal Society of Chemistry. The magazine addresses current events in world of chemistry including research, international business news and government policy as it affects the chemical science community, plus the best product...
: Osmium

