
Neutron cross-section
Encyclopedia
In nuclear
and particle physics
, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron
and a target nucleus. In conjunction with the neutron flux
, it enables the calculation of the reaction rate, for example to derive the thermal power
of a nuclear power plant
. The standard unit for measuring the cross section is the barn
, which is equal to 10-28 m2 or 10-24 cm2.
and, to a lesser extent, of:
-2 (referred to as deuterium
) is much smaller than that of common hydrogen-1 . This is the reason why some reactors use heavy water
(in which most of the hydrogen is deuterium) instead of ordinary light water
as moderator
: fewer neutrons are lost by capture inside the medium, hence enabling the use of natural uranium
instead of enriched uranium
. This is the principle of a CANDU reactor
.

dependence of the scattering cross-section and for a natural sample, presence of different isotope
s of the same element in the sample.
Since neutrons interact with the nuclear potential, the scattering cross-section varies with the atomic number
of the element in question. A very prominent example is hydrogen
and its isotope deuterium
. The total cross-section for hydrogen is over 10 times that of deuterium, mostly due to the large incoherent scattering length of hydrogen. Metals tend to be rather transparent to neutrons, aluminum and zirconium
being the two best examples of this.
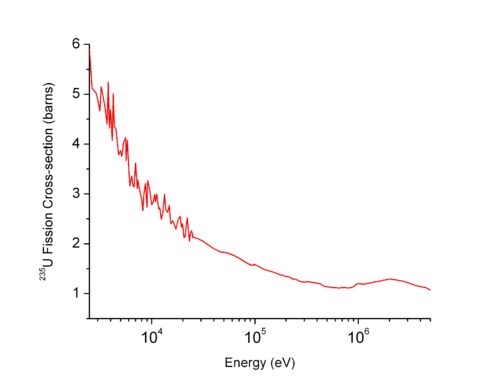 For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either null (the energy for which the cross section becomes significant is called threshold energy
For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either null (the energy for which the cross section becomes significant is called threshold energy
) or, on the contrary, much larger than the cross section at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group). See here for more details.
As an example, the plot on the right shows that the fission
cross section of the uranium 235 is low at high neutron energies but becomes higher at low energies. Such physical constraint explains why most of the operational nuclear reactors use a neutron moderator
to reduce the energy of the neutron and thus increase the probability of fission, essential to produce energy and sustain the chain reaction
.

Where σ is the cross section at temperature T and σ0 the cross section at temperature T0 (T and T0 in Kelvin
)

Noting n the number of particles per unit volume, there are n V particles in the volume V, which will, per definition of V, undergo a reaction. Noting r the reaction rate
onto one target, it gives:

It follows directly from the definition of the neutron flux
Φ = n v:

Assuming that there is not one but N targets per unit volume, the reaction rate R per unit volume is:

Knowing that the typical nuclear radius r is of the order of 10-12 cm, the expected nuclear cross section is of the order of π r2 or roughly 10-24 cm2 (thus justifying the definition of the barn
). However, if measured experimentally (σ = R / (Φ N), the experimental cross sections vary enormously. As an example, for slow neutrons absorbed by the (n, γ) reaction the cross section in some cases is as much as 1,000 barns, while the cross sections for transmutations by gamma-ray absorption are in the neighborhood of 0.001 barn (See here for more example of cross sections).
The “nuclear cross section” is consequently a purely conceptual quantity representing how big should be the atom to be consistent with this simple mechanical model.

Where σ(E) is the continuous cross section, Φ(E) the differential flux and N the target atom density.
In order to obtain a formulation equivalent to the mono energetic case, an average cross section is defined:

Where Φ= Φ(E) dE is the integral flux.
Φ(E) dE is the integral flux.
Using the definition of the integral flux Φ and the average cross section σ, the same formulation as before is found:


where N is the atomic density of target.
Therefore, since the cross section can be expressed in cm2 and the density in cm-3, the macroscopic cross section is usually expressed in cm-1. Using equation derived in #Link to reaction rate and interpretation, the reaction rate per unit volume R can be derived using only the neutron flux Φ and the macroscopic cross section Σ:

The total length L that non perturbed particles travel during a time interval dt in a volume dV is simply the product of the length l covered by each particle during this time with the number of particles N in this volume:

Noting v the speed of the particles and n is the number of particles per unit volume:


It follows:

Using the definition of the neutron flux
Φ

It follows:

This average length L is however valid only for unperturbed particles. To account for the interactions, L is divided by the total number of reactions R to obtain the average length between each collision λ:

From #Microscopic versus macroscopic cross section:

It follows:

where λ is the mean free path and Σ is the macroscopic cross section.
-8 and beryllium
-12 form natural stopping points on the table of isotopes for hydrogen
fusion
it is believed that all of the higher elements are formed in very hot stars where higher orders of fusion predominate. A star like the Sun
produces energy
by the fusion of simple H-1 into helium
-4 through a series of reactions. It is believed that when the inner core exhausts its H-1 fuel the sun will contract, slightly increasing its core temperature until He-4 can fuse and become the main fuel supply. Pure He-4 fusion leads to Be-8, which decays back to 2 He-4 therefore the He-4 must fuse with isotopes either more or less massive than itself to result in an energy producing reaction. When He-4 fuses with H-2
or H-3
it forms stable isotopes Li-6 and Li-7 respectively. The higher order isotopes between Li-8 and C-12 are synthesized by similar reactions between hydrogen, helium and lithium isotopes.
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
and particle physics
Particle physics
Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics...
, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
and a target nucleus. In conjunction with the neutron flux
Neutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
, it enables the calculation of the reaction rate, for example to derive the thermal power
Power (physics)
In physics, power is the rate at which energy is transferred, used, or transformed. For example, the rate at which a light bulb transforms electrical energy into heat and light is measured in watts—the more wattage, the more power, or equivalently the more electrical energy is used per unit...
of a nuclear power plant
Nuclear power plant
A nuclear power plant is a thermal power station in which the heat source is one or more nuclear reactors. As in a conventional thermal power station the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity.Nuclear power plants are usually...
. The standard unit for measuring the cross section is the barn
Barn (unit)
A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the...
, which is equal to 10-28 m2 or 10-24 cm2.
Parameters of interest
The neutron cross section, and therefore the probability of an interaction, depends on:- the target type (hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, uraniumUraniumUranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
…), - the type of nuclear reactionNuclear reactionIn nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce products different from the initial particles...
(scattering, fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
…). - the incident particle energyNeutron temperatureThe neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is...
, also called speed or temperature (thermal, fast…),
and, to a lesser extent, of:
- its relative angle between the incident neutron and the target nuclide,
- the target nuclide temperature.
Target type dependence
The neutron cross section is defined for a given type of target particle. For example, the capture cross section of hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
-2 (referred to as deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
) is much smaller than that of common hydrogen-1 . This is the reason why some reactors use heavy water
Heavy water reactor
A pressurised heavy water reactor is a nuclear power reactor, commonly using unenriched natural uranium as its fuel, that uses heavy water as its coolant and moderator. The heavy water coolant is kept under pressure in order to raise its boiling point, allowing it to be heated to higher...
(in which most of the hydrogen is deuterium) instead of ordinary light water
Light water reactor
The light water reactor is a type of thermal reactor that uses normal water as its coolant and neutron moderator. Thermal reactors are the most common type of nuclear reactor, and light water reactors are the most common type of thermal reactor...
as moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
: fewer neutrons are lost by capture inside the medium, hence enabling the use of natural uranium
Natural uranium
Natural uranium refers to refined uranium with the same isotopic ratio as found in nature. It contains 0.7 % uranium-235, 99.3 % uranium-238, and a trace of uranium-234 by weight. In terms of the amount of radioactivity, approximately 2.2 % comes from uranium-235, 48.6 % uranium-238, and 49.2 %...
instead of enriched uranium
Enriched uranium
Enriched uranium is a kind of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Natural uranium is 99.284% 238U isotope, with 235U only constituting about 0.711% of its weight...
. This is the principle of a CANDU reactor
CANDU reactor
The CANDU reactor is a Canadian-invented, pressurized heavy water reactor. The acronym refers to its deuterium-oxide moderator and its use of uranium fuel...
.
Type of reaction dependence
The likelihood of interaction between an incident neutron and a target nuclide, independent of the type of reaction, is expressed with the help of the total cross section σT. However, it may be useful to know if the incoming particle bounces off the target (and therefore continue travelling after the interaction) or disappears after the reaction. For that reason, the scattering and absorption cross sections σS and σA are defined and the total cross section is simply the sum of the two partial cross sections :
Absorption cross section
If the neutron is absorbed when approaching the nuclide, the atomic nucleus moves up on the table of isotopes by one position. For instance, U-235 becomes U-236* with the * indicating the nucleus is highly energized. This energy has to be released and the release can take place through any of several mechanisms.- The simplest way for the release to occur is for the neutron to be ejected by the nucleus. If the neutron is emitted immediately, it acts the same as in other scattering events.
- The nucleus may emit gamma radiation.
- The nucleus may β- decay, where a neutron is converted into a proton, an electron and an electron-type antineutrino (the antiparticle of the neutrino)
- About 81% of the U-236* nuclei are so energized that they undergo fission, releasing the energy as kinetic motion of the fission fragments, also emitting between one and five free neutrons.
- Nuclei that undergo fission as their predominant decay method after neutron capture include U-233, U-235, U-237, Pu-239, Pu-241.
- Nuclei that predominantly absorb neutrons and then emit Beta particle radiation lead to these isotopes, e.g., Th-232 absorbs a neutron and becomes Th-233*, which emits a Beta particle and becomes Pa-233, which emits another Beta particle to become U-233.
- Isotopes that undergo Beta emission transmute from one element to another element, those that undergo gamma or X-ray emission don't change in element or isotope.
Scattering cross-section
The scattering cross-section can be further subdivided into coherent scattering and incoherent scattering, which is caused by the spinSpin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
dependence of the scattering cross-section and for a natural sample, presence of different isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of the same element in the sample.
Since neutrons interact with the nuclear potential, the scattering cross-section varies with the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of the element in question. A very prominent example is hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and its isotope deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
. The total cross-section for hydrogen is over 10 times that of deuterium, mostly due to the large incoherent scattering length of hydrogen. Metals tend to be rather transparent to neutrons, aluminum and zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
being the two best examples of this.
Incident particle energy dependence

Threshold energy
In particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles must have when they collide. The threshold energy is always greater than or equal to the rest energy of the desired particle...
) or, on the contrary, much larger than the cross section at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group). See here for more details.
As an example, the plot on the right shows that the fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
cross section of the uranium 235 is low at high neutron energies but becomes higher at low energies. Such physical constraint explains why most of the operational nuclear reactors use a neutron moderator
Neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, thereby turning them into thermal neutrons capable of sustaining a nuclear chain reaction involving uranium-235....
to reduce the energy of the neutron and thus increase the probability of fission, essential to produce energy and sustain the chain reaction
Chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events....
.
Target temperature dependence
Cross sections are usually measured at 20°C. To account for the dependence with temperature of the medium (viz. the target), the following formula is used :
Where σ is the cross section at temperature T and σ0 the cross section at temperature T0 (T and T0 in Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
)
Link to reaction rate and interpretation
Let us imagine a spherical target (in grey in the figure) and a beam of particles (in blue) “flying” at speed v (vector in black) in the direction of the target. We want to know how many particles impact it during time interval dt. To achieve it, the particles have to be in the cylinder in green in the figure (volume V). The base of the cylinder is the geometrical cross section of the target perpendicular to the beam (surface σ in red) and its height the length travelled by the particles during dt (length v dt):
Noting n the number of particles per unit volume, there are n V particles in the volume V, which will, per definition of V, undergo a reaction. Noting r the reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
onto one target, it gives:

It follows directly from the definition of the neutron flux
Neutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
Φ = n v:

Assuming that there is not one but N targets per unit volume, the reaction rate R per unit volume is:

Knowing that the typical nuclear radius r is of the order of 10-12 cm, the expected nuclear cross section is of the order of π r2 or roughly 10-24 cm2 (thus justifying the definition of the barn
Barn
A barn is an agricultural building used for storage and as a covered workplace. It may sometimes be used to house livestock or to store farming vehicles and equipment...
). However, if measured experimentally (σ = R / (Φ N), the experimental cross sections vary enormously. As an example, for slow neutrons absorbed by the (n, γ) reaction the cross section in some cases is as much as 1,000 barns, while the cross sections for transmutations by gamma-ray absorption are in the neighborhood of 0.001 barn (See here for more example of cross sections).
The “nuclear cross section” is consequently a purely conceptual quantity representing how big should be the atom to be consistent with this simple mechanical model.
Continuous versus average cross section
Cross sections depend strongly on the incoming particle speed. In the case of a beam with multiple particle speeds, the reaction rate R is integrated over the whole range of energy:
Where σ(E) is the continuous cross section, Φ(E) the differential flux and N the target atom density.
In order to obtain a formulation equivalent to the mono energetic case, an average cross section is defined:

Where Φ=
 Φ(E) dE is the integral flux.
Φ(E) dE is the integral flux.Using the definition of the integral flux Φ and the average cross section σ, the same formulation as before is found:

Microscopic versus macroscopic cross section
Up to now, the cross section referred to in this article corresponds to the microscopic cross section σ. However, it is possible to define the macroscopic cross section Σ which corresponds to the total “equivalent area” of all target particles per unit volume:
where N is the atomic density of target.
Therefore, since the cross section can be expressed in cm2 and the density in cm-3, the macroscopic cross section is usually expressed in cm-1. Using equation derived in #Link to reaction rate and interpretation, the reaction rate per unit volume R can be derived using only the neutron flux Φ and the macroscopic cross section Σ:

Mean free path
The aim of this paragraph is to evaluate the “mean free path” λ of a random particle, that is to say the average length travelled between two interactions.The total length L that non perturbed particles travel during a time interval dt in a volume dV is simply the product of the length l covered by each particle during this time with the number of particles N in this volume:

Noting v the speed of the particles and n is the number of particles per unit volume:


It follows:

Using the definition of the neutron flux
Neutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
Φ

It follows:

This average length L is however valid only for unperturbed particles. To account for the interactions, L is divided by the total number of reactions R to obtain the average length between each collision λ:

From #Microscopic versus macroscopic cross section:

It follows:

where λ is the mean free path and Σ is the macroscopic cross section.
Within stars
Because lithiumLithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
-8 and beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
-12 form natural stopping points on the table of isotopes for hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
it is believed that all of the higher elements are formed in very hot stars where higher orders of fusion predominate. A star like the Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
produces energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
by the fusion of simple H-1 into helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
-4 through a series of reactions. It is believed that when the inner core exhausts its H-1 fuel the sun will contract, slightly increasing its core temperature until He-4 can fuse and become the main fuel supply. Pure He-4 fusion leads to Be-8, which decays back to 2 He-4 therefore the He-4 must fuse with isotopes either more or less massive than itself to result in an energy producing reaction. When He-4 fuses with H-2
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
or H-3
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
it forms stable isotopes Li-6 and Li-7 respectively. The higher order isotopes between Li-8 and C-12 are synthesized by similar reactions between hydrogen, helium and lithium isotopes.
Typical cross sections
In the following, some cross sections which are of importance in a nuclear reactor are given. The thermal cross-section is averaged using a Maxwellian spectrum and the fast cross section is averaged using the uranium-235 fission spectrum. The cross sections are taken from the Jeff-3.1.1 library using Janis software .| Thermal cross section (barn) | Fast cross section (barn) | ||||||
| Scattering | Capture | Fission | Scattering | Capture | Fission | ||
| Moderator | H-1 | 2E+1 | 2E-1 | - | 4E+0 | 4E-5 | - |
| H-2 | 4E+0 | 3E-4 | - | 3E+0 | 7E-6 | - | |
| C (nat) | 5E+0 | 2E-3 | - | 2E+0 | 1E-5 | - | |
| Structural materials, others | Zr-90 | 5E+0 | 6E-3 | - | 5E+0 | 6E-3 | - |
| Fe-56 | 1E+1 | 2E+0 | - | 2E+1 | 3E-3 | - | |
| Cr-52 | 3E+0 | 5E-1 | - | 3E+0 | 2E-3 | - | |
| Ni-58 | 2E+1 | 3E+0 | - | 3E+0 | 8E-3 | - | |
| 0-16 | 4E+0 | 1E-4 | - | 3E+0 | 3E-8 | - | |
| Absorber | B-10 | 2E+0 | 2E+3 | - | 2E+0 | 4E-1 | - |
| Cd-113 | 1E+2 | 3E+4 | - | 4E+0 | 5E-2 | - | |
| Xe-135 | 4E+5 | 2E+6 | - | 5E+0 | 8E-4 | - | |
| In-115 | 2E+0 | 1E+2 | - | 4E+0 | 2E-1 | - | |
| Fuel | U-235 | 1E+1 | 6E+1 | 3E+2 | 4E+0 | 9E-2 | 1E+0 |
| U-238 | 9E+0 | 2E+0 | 2E-5 | 5E+0 | 7E-2 | 3E-1 | |
| Pu-239 | 8E+0 | 4E-2 | 7E-2 | 5E+0 | 5E-2 | 2E+0 | |

