Nitrilimine
Encyclopedia
Nitrilimines or nitrile amides are a class of organic compound
s sharing a common functional group
with the general structure R-CN-NR corresponding to an amine
bonded to the N-terminus of a nitrile
. The dominant structure for the parent compound nitrilimine is that of the propargyl
-like 1 in scheme 1 with a C-N triple bond
and with a formal positive charge on nitrogen and two lone pair
s and a formal negative charge on the terminal nitrogen. Other structures such as hypervalent 2, allene
-like 3, allylic 4 and carbene
5 are of lesser relevance.
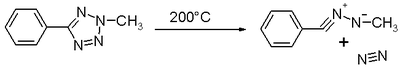
 Nitrilimines were first observed in the thermal decomposition of 2-tetrazole
Nitrilimines were first observed in the thermal decomposition of 2-tetrazole
s releasing nitrogen:
 Nitrilimines are linear 1,3-dipole
Nitrilimines are linear 1,3-dipole
s in 1,3-dipolar cycloaddition
s and are represented by structures 1 and 3. With alkyne
s they generate pyrazole
s.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s sharing a common functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
with the general structure R-CN-NR corresponding to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
bonded to the N-terminus of a nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
. The dominant structure for the parent compound nitrilimine is that of the propargyl
Propargyl
In organic chemistry, propargyl is an alkyl functional group of 2-propynyl with the structure HC≡C−CH2−, derived from the alkyne propyne.The term propargylic refers to a saturated position on a molecular framework next to an alkynyl group...
-like 1 in scheme 1 with a C-N triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
and with a formal positive charge on nitrogen and two lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s and a formal negative charge on the terminal nitrogen. Other structures such as hypervalent 2, allene
Allene
An allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
-like 3, allylic 4 and carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
5 are of lesser relevance.

Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen and one carbon atom . The simplest is tetrazole itself, CN4H2. They are unknown in nature...
s releasing nitrogen:

1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms...
s in 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
s and are represented by structures 1 and 3. With alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s they generate pyrazole
Pyrazole
Pyrazole refers both to the class of simple aromatic ring organic compounds of the heterocyclic diazole series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions, and to the unsubstituted parent compound...
s.

