
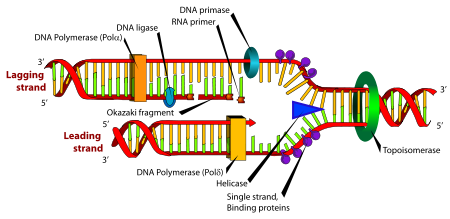
Okazaki fragment
Encyclopedia
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
that are formed on the lagging strand
Replication fork
The replication fork is a structure that forms within the nucleus during DNA replication. It is created by helicases, which break the hydrogen bonds holding the two DNA strands together. The resulting structure has two branching "prongs", each one made up of a single strand of DNA...
during DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
. They are between 1,000 to 2,000 nucleotides long in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
and are between 100 to 200 nucleotides long in eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s.
On the leading strand DNA replication proceeds continuously along the DNA molecule as the parent double-stranded DNA is unwound. But on the lagging strand the new DNA is made in installments, which are later joined together by a DNA ligase
DNA ligase
In molecular biology, DNA ligase is a specific type of enzyme, a ligase, that repairs single-stranded discontinuities in double stranded DNA molecules, in simple words strands that have double-strand break . Purified DNA ligase is used in gene cloning to join DNA molecules together...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
. This is because the enzymes
DNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
that synthesise the new DNA can only work in one direction along the parent DNA molecule. On the leading strand this route is continuous, but on the lagging strand it is discontinuous.
They were originally discovered in 1966 by Kiwako Sakabe and Reiji Okazaki
Reiji Okazaki
was a Japanese molecular biologist known for his research in DNA replication and especially for describing the role of so-called Okazaki fragments which he discovered working with his wife Tsuneko Okazaki in 1968.- Biography :...
in their research on DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
of Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
. They were further investigated by them and their colleagues through their research including the study on bacteriophage
Bacteriophage
A bacteriophage is any one of a number of viruses that infect bacteria. They do this by injecting genetic material, which they carry enclosed in an outer protein capsid...
DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
.
Experiments
The work of TsunekoTsuneko Okazaki
is a Japanese scientist known for her discovery and research of Okazaki fragments along with her husband Reiji.Okazaki graduated from Nagoya University, School of Science in 1956.-References:...
and Reiji Okazaki
Reiji Okazaki
was a Japanese molecular biologist known for his research in DNA replication and especially for describing the role of so-called Okazaki fragments which he discovered working with his wife Tsuneko Okazaki in 1968.- Biography :...
provided experimental evidence supporting the hypothesis that DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
is a discontinuous process. Previously, it was commonly accepted that replication was continuous in both the 3’ to 5’ and 5’ to 3’ directions. 3’ and 5’ are specifically numbered carbons on the deoxyribose ring in nucleic acids, and refer to the orientation or directionality
Directionality (molecular biology)
Directionality, in molecular biology and biochemistry, is the end-to-end chemical orientation of a single strand of nucleic acid. The chemical convention of naming carbon atoms in the nucleotide sugar-ring numerically gives rise to a 5′-end and a 3′-end...
of a strand. In 1967, the Okazakis and their colleagues suggested that there is no found mechanism that showed continuous replication in the 3’ to 5’ direction, only 5’ to 3’ using DNA polymerase
DNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
, a replication enzyme. The team hypothesized that if discontinuous replication was used, short strands of DNA, synthesized at the replicating point, could be attached in the 5’ to 3’ direction to the older strand.
To distinguish the method of replication used by DNA experimentally, the team pulse-labeled
Pulse labelling
Pulse labelling is a biochemistry technique of identifying the target molecule presence by inclusion of a pulse of a radioactive compound. This is mainly done to identify the stage at which the messenger RNA is being produced in a cell. In this the pulse of 3H atom is pulsed for a period of around...
newly replicated areas of Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
chromosomes, denatured, and extracted the DNA. A large amount of radioactive short units meant that the replication method was likely discontinuous. The hypothesis was further supported by the discovery of polynucleotide ligase
Ligase
In biochemistry, ligase is an enzyme that can catalyse the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group dependent to one of the larger molecules...
, an enzyme that links short DNA strands together.
In 1968, the Okazakis gathered additional evidence of nascent DNA strands. They hypothesized that if discontinuous replication, involving short DNA chains linked together by polynucleotide ligase
Ligase
In biochemistry, ligase is an enzyme that can catalyse the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group dependent to one of the larger molecules...
, is the mechanism used in DNA synthesis, then “newly synthesized short DNA chains would accumulate in the cell under conditions where the function of ligase is temporarily impaired.” E.coli were infected with Bacteriophage T4 that produce temperature-sensitive polynucleotide ligase. The cells infected with the T4 Phages accumulated a large amount of short, newly synthesized DNA chains, as predicted in the hypothesis, when exposed to high temperatures. This experiment further supported the Okazakis’ hypothesis of discontinuous replication and linkage by polynucleotide ligase. It disproved the notion that short chains were produced during the extraction process as well.
The Okazakis’ experiments provided extensive information on the replication process of DNA and the existence of short, newly synthesized DNA chains that later became known as Okazaki fragments.
Pathways
There are two pathways that have been proposed to process Okazaki fragments.In the first pathway, only the nuclease FEN1, which cleaves the short flaps immediately when they form, is involved. While this pathway can process basically all flaps, an issue with this pathway is that some flaps may escape cleavage and thus become long. These flaps then bind to replication protein A (RPA) which inhibits FEN1 cleavage.
The second pathway thus becomes involved and is able to utilize both FEN1 and Dna2 nucleases to process the long flaps. Dna2 can cleave the RPA bound flap as it is able to displace to RPA, while creating a flat to which RPA cannot bind. Then, FEN1 will complete the cleavage of the flap. Dna2 is a key part of this process. Without the Dna2, the RPA bound flaps could not be processed which would ultimately lead to cell instability. The Pif1 helicase is also involved in this pathway as it aids creation of long flaps. Without the Pif1 helicase, the flaps would not become long enough to need cleavage by Dna2.
Recently, it has been suggested that an alternative pathway for Okazaki fragment processing exists. This alternative pathway occurs when the Pif1 helicase removes entire Okazaki fragments initiated by fold back flaps.
Alternate pathway
Until recently, there were only two known pathways to process Okazaki fragments. However, current investigations have concluded that a new pathway for Okazaki fragmentation and DNA replication exists. This alternate pathway involves the enzymes Pol δ with Pif1 which perform the same flap removal process as Polδ and FEN1.Primase
PrimasePrimase
DNA primase is an enzyme involved in the replication of DNA.Primase catalyzes the synthesis of a short RNA segment called a primer complementary to a ssDNA template...
adds RNA primers onto the lagging strand, which allows synthesis of Okazaki fragments from 5’ to 3’. However, Primase
Primase
DNA primase is an enzyme involved in the replication of DNA.Primase catalyzes the synthesis of a short RNA segment called a primer complementary to a ssDNA template...
creates RNA primers at a much lower rate than that at which DNA polymerase
DNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
synthesizes DNA on the leading strand. DNA polymerase on the lagging strand also has to be continually recycled to construct Okazaki fragments following RNA primers. This makes the speed of lagging strand synthesis much lower than that of the leading strand. To solve this, primase acts as a temporary stop signal, briefly halting the progression of the replication fork
Replication fork
The replication fork is a structure that forms within the nucleus during DNA replication. It is created by helicases, which break the hydrogen bonds holding the two DNA strands together. The resulting structure has two branching "prongs", each one made up of a single strand of DNA...
during DNA replication. This molecular process prevents the leading strand from overtaking the lagging strand.
DNA polymerase δ
Following creation of RNA primers by Primase on the lagging strand, DNA polymerase δ synthesizes Okazaki fragments. DNA polymerase δ also carries out a 3’ to 5’ exonucleaseExonuclease
Exonucleases are enzymes that work by cleaving nucleotides one at a time from the end of a polynucleotide chain. A hydrolyzing reaction that breaks phosphodiester bonds at either the 3’ or the 5’ end occurs. Its close relative is the endonuclease, which cleaves phosphodiester bonds in the middle ...
role, proofreading newly synthesized DNA strands during DNA replication. When the polymerase encounters an erroneous base pair, it removes one of the nucleotides and replaces it with a correct one. A third function of DNA polymerase δ is to supplement FEN1/RAD27 5’ Flap Endonuclease activity. This includes preventing and removing the strand displacement of 5’ flaps, and creating ligatable nicks at the border of Okazaki fragments.
DNA ligase I
During lagging strand synthesis, DNA ligaseDNA ligase
In molecular biology, DNA ligase is a specific type of enzyme, a ligase, that repairs single-stranded discontinuities in double stranded DNA molecules, in simple words strands that have double-strand break . Purified DNA ligase is used in gene cloning to join DNA molecules together...
I connects the Okazaki fragments, following replacement of the RNA primer
Primer (molecular biology)
A primer is a strand of nucleic acid that serves as a starting point for DNA synthesis. They are required for DNA replication because the enzymes that catalyze this process, DNA polymerases, can only add new nucleotides to an existing strand of DNA...
s with DNA nucleotides by DNA polymerase δ. Okazaki fragments that are not ligated could cause double-strand-breaks, which cleaves the DNA. Since only a small number of double-strand breaks are tolerated, and only a small number can be repaired, enough ligation failures could be lethal to the cell. Further research implicates the supplementary role of Proliferating cell nuclear antigen (PCNA) to DNA Ligase I’s function of joining Okazaki fragments. When the PCNA binding site on DNA Ligase I is inactive, DNA Ligase I’s ability to connect Okazaki fragments was severely impaired. Thus, a proposed mechanism follows: after a PCNA-DNA polymerase δ complex synthesizes Okazaki fragments, the DNA polymerase δ is released. Then, DNA Ligase I binds to the PCNA, which is clamped to the nicks of the lagging strand, and catalyzes the formation of phosphodiester bonds.
Flap endonuclease 1
Flap endonuclease 1 (FEN1) is responsible for processing Okazaki fragments. It works with DNA polymerase to remove the RNA primer of an Okazaki fragment and can remove the 5’ ribonucleotide and 5’ flaps when DNA polymeraseDNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
displaces the strands during lagging strand synthesis. The removal of these flaps involves a process called nick translation and creates a nick for ligation. Thus, FEN1’s function is necessary to Okazaki fragment maturation in forming a long continuous DNA strand. Likewise, during DNA base repair, the damaged nucleotide is displaced into a flap and subsequently removed by FEN1.
Dna2 endonuclease
In the presence of a single stranded DNA-binding protein RPA, the DNA 5’ flaps become too long, and the nicks no longer fit as substrate for FEN1. This prevents the FEN1 from removing the 5′-flaps. Thus, Dna2’s role is to reduce the 3′ end of these fragments, making it possible for FEN1 to cut the flaps, and the Okazaki fragment maturation more efficient.Biological function
Although synthesis of the lagging strand involves only half the DNA in the nucleus, the complexity associated with processing Okazaki fragments is about twice that required to synthesize the leading strand. Even in small species such as yeast, Okazaki fragment maturation happens approximately a million times during a single round of DNA replication. Processing of Okazaki fragments is therefore very common and crucial for DNA replication and cell proliferation.During this process, RNA and DNA primers are removed, allowing the Okazaki fragments to attach to the lagging DNA strand. While this process seems quite simple and repetitive, defects in Okazaki fragment maturation can cause DNA strand breakage which can cause varying forms of “chromosome aberrations”. Severe defects of Okazaki fragment maturation may halt DNA replication and induce cell death. However, while subtle defects do not affect growth, they do result in future varying forms of genome instabilities. Based on the dangers associated with a failure in the DNA process, Okazaki fragments maintain our evolutionary development.
Okazaki fragments in prokaryotes and eukaryotes
DNA molecules in eukaryotes differ from the circular molecules of prokaryotes in that they are larger and usually have multiple origins of replication. This means that each eukaryotic chromosome is composed of many replicating units of DNADNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
with multiple origins of replication. In comparison, the prokaryotic E. coli chromosome has only a single origin of replication. In eukaryotes, these replicating forks, which are numerous all along the DNA, form "bubbles" in the DNA during replication. The replication fork forms at a specific point called autonomously replicating sequences (ARS). Eukaryotes have a clamp loader complex and a six-unit clamp called the proliferating cell nuclear antigen. The efficient movement of the replication fork also relies critically on the rapid placement of sliding clamps at newly primed sites on the lagging DNA strand by ATP-dependent clamp loader complexes. This means that the piecewise generation of Okazaki fragments can keep up with the continuous synthesis of DNA on the leading strand. These clamp loader complexes are characteristic of all eukaryotes and separate some of the minor differences in the synthesis of Okazaki fragments in prokaryotes and eukaryotes.
The lengths of Okazaki fragments in prokaryotes and eukaryotes are different as well. Prokaryotes have Okazaki fragments that are quite longer than those of eukaryotes. Eukaryotes typically have Okazaki fragments that are 100 to 200 nucleotides long, whereas prokaryotic E. Coli can be 2,000 nucleotides long. Okazaki fragments are also produced much quicker in prokaryotes since prokaryotic cells contain less genetic material. The complexity of the DNA in eukaryotes calls for a longer production time of the Okazaki fragments.
Medical concepts associated with Okazaki fragments
To test the effects of the protein mutations on living organisms, researchers genetically altered lab mice to be homozygous for another mutation in protein related to DNA replication, flap endonuclease 1, or FEN1. The results varied based on the specific gene alterations. Homozygous knockout mutant mice experienced a “failure of cell proliferation” and “early embryonic lethality” (27). Mice with mutation F343A and F344A (also known as FFAA) died directly after birth due to complications including pancytopenia
Pancytopenia
Pancytopenia is a medical condition in which there is a reduction in the number of red and white blood cells, as well as platelets.If only two parameters from the full blood count are low, the term bicytopenia can be used...
and pulmonary hypoplasia
Hypoplasia
Hypoplasia is underdevelopment or incomplete development of a tissue or organ. Although the term is not always used precisely, it properly refers to an inadequate or below-normal number of cells. Hypoplasia is similar to aplasia, but less severe. It is technically not the opposite of hyperplasia...
. This is because the FFAA mutation keeps FEN1 from interacting with PCNA (proliferating cell nuclear antigen), consequently not allowing it to complete its purpose during Okazaki fragment maturation. Under careful observation, cells homozygous for FFAA FEN1 mutations seem to display only partial defects in maturation, meaning mice heterozygous for the mutation would be able to survive into adulthood, despite sustaining multiple small nicks in their genomes. Inevitably however, these nicks prevent future DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
because the break causes the replication fork to collapse and causes double strand breaks in the actual DNA sequence. In time, these nicks also cause full chromosome breaks, which could lead to severe mutations and cancers. Other mutations have been implemented with altered versions of Polymerase α, leading to similar results.
Engineering concepts associated with Okazaki fragments
Due Okazaki fragment’s importance and purpose in DNA replication, bioengineers are using these pieces of DNA in their research. One study, published in Science on March 17, 2000, stated that the specific start and stop locations of bidirectional DNA synthesis at a human gene called Lamin B2 were discovered using DNA base exploration and information about Okazaki fragment synthesis. Based on the relative position of Okazaki fragments to the replication fork during lagging stand synthesis as well as the average length of Okazaki fragments during replication, the scientists were able to pinpoint nucleotide 3933 as the start of bidirectional synthesis. The information was, “deriving from the linking to the initiated ori of four subsequent Okazaki fragments having lengths of 143, 144, 65, and 80 nucleotides from the first to the fourth, respectively”.Another study investigated the formation of Okazaki fragments in wild-type bacteria cells. In strains of Escherichia coli, a mutation in the sof locus, a specific location of a gene on a chromosome, resulted in hyper recombination and short DNA strands. Scientists say that the mutated gene is defective, affecting an enzyme called deoxyuridinetriphosphate diphosphohydrolase (or dUTPase), which can no longer catalyze the hydrolysis of dUTP. This mutation also decreases the amount of dUTPase in the organism, somehow increasing the amount of uracil that is being incorporated into the DNA. Because uracil is not one of the four nucleotides associated with DNA (replaced by Thymine), it must be removed as an error by the excision-repair process and replaced. Rapid removal of uracil because of increased incorporation into DNA can lead to the accumulation of short DNA fragments, which may also lead to the creation of Okazaki fragments. Learning how Okazaki fragments originate in bacteria such as E. coli allows scientists and engineers to better understand the process of DNA replication and the effects of pinpoint mutations.
Although many mutations associated with Okazaki fragments can result in cancer, studies have shown that the alteration of Okazaki fragments in nucleoside analogues such as cytarabine
Cytarabine
Cytarabine, or cytosine arabinoside, is a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia and non-Hodgkin lymphoma. It is also known as Ara-C...
can lead to anti-cancer activities. For instance, cytarabine is an anti-leukemic agent that inhibits the replication of lagging stand DNA. When this agent was substituted for deoxycytidine during the replication of the SV-40 viral genome, the bending of the DNA duplex region (DDR), and the RNA-DNA hybrid duplex region (HDR) increased from 22 degrees to 41 degrees. This increased helical bending of Okazaki fragments may contribute to cytarabine's ability to inhibit the replication of lagging strand DNA and have anti-cancer properties.

