Organocatalysis
Encyclopedia
In organic chemistry
, the term Organocatalysis (a concatenation
of the terms "organic" and "catalyst") refers to a form of catalysis
, whereby the rate of a chemical reaction
is increased by an organic catalyst
referred to as an "organocatalyst" consisting of carbon
, hydrogen
, sulfur
and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer
for enzyme
s due to their comparable effects on reaction rates and forms of catalysis involved.
Organocatalysts which display secondary amine
functionality can be described as performing either enamine
catalysis (by forming catalytic quantities of an active enamine nucleophile
) or iminium
catalysis (by forming catalytic quantities of an activated iminium electrophile). This mechanism is typical for covalent organocatalysis. Covalent binding of substrate normally requires high catalyst loading (for proline-catalysis typically 20-30 mol%).
Noncovalent interactions such as hydrogen-bonding facilitates low catalyst loadings (down to 0.001 mol%).
Organocatalysis offers several advantages. There is no need for metal-based catalysis thus making a contribution to green chemistry
. In this context, simple organic acids have been used as catalyst for the modification of cellulose in water on multi-ton scale. When the organocatalyst is chiral
an avenue is opened to asymmetric catalysis, for example the use of proline
in aldol reaction
s,
used in the Knoevenagel condensation
, DMAP
used in esterfications and DABCO
used in the Baylis-Hillman reaction. Thiazolium salts are employed in the Stetter reaction
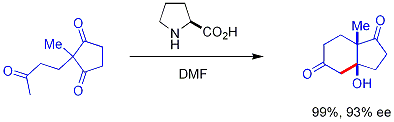
. These catalysts and reactions have a long history but current interest in organocatalysis is focused on asymmetric catalysis with chiral catalysts and this particular branch is called asymmetric organocatalysis or enantioselective organocatalysis . A pioneering reaction developed in the 1970s is called the Hajos-Parrish reaction
:
In this reaction, naturally occurring chiral proline
is the chiral catalyst in an Aldol reaction
. The starting material is an achiral triketone
and it requires just 3% of proline to obtain the reaction product, a ketol in 93% enantiomeric excess
. This is the first example of an amino acid-catalyzed asymmetric aldol reaction.
The asymmetric synthesis of the Wieland-Miescher ketone
(1985) is also based on proline and another early application was one of the transformations in the total synthesis
of Erythromycin
by Robert B. Woodward (1981).
Many chiral organocatalysts are an adaptation of chiral ligand
s (which together with a metal center also catalyze asymmetric reactions) and both concepts overlap to some degree.
Examples of asymmetric reactions involving organocatalysts are:
phenylalanine
in two chemical steps (amidation with methylamine
followed by condensation reaction
with acetone
) which leave the chirality intact :
This catalyst works by forming a iminium ion with carbonyl
groups of α,β-unsaturated aldehydes (enals) and enone
s in a rapid chemical equilibrium
. This iminium activation is similar to activation of carbonyl groups by a Lewis acid
and both catalysts lower the substrate's LUMO
:
The transient iminium intermediate is chiral which is transferred to the reaction product via chiral induction. The catalysts have been used in Diels-Alder reaction
s, Michael additions, Friedel-Crafts alkylations, transfer hydrogenation
s and epoxidations.
One example is the asymmetric synthesis of the drug warfarin
(in equilibrium with the hemiketal) in a Michael addition of 4-hydroxycoumarin and benzylideneacetone
:
A recent exploit is the vinyl
alkylation of crotonaldehyde
with an organotrifluoroborate salt
:
For other examples of its use: see organocatalytic transfer hydrogenation and asymmetric Diels-Alder reactions.
or the thiourea
moiety. These catalytically effective (thio)urea derivatives termed (thio)urea organocatalysts provide explicit double hydrogen-bonding interactions to coordinate and activate H-bond accepting substrates.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the term Organocatalysis (a concatenation
Concatenation
In computer programming, string concatenation is the operation of joining two character strings end-to-end. For example, the strings "snow" and "ball" may be concatenated to give "snowball"...
of the terms "organic" and "catalyst") refers to a form of catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, whereby the rate of a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
is increased by an organic catalyst
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
referred to as an "organocatalyst" consisting of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer
Misnomer
A misnomer is a term which suggests an interpretation that is known to be untrue. Such incorrect terms sometimes derive their names because of the form, action, or origin of the subject becoming named popularly or widely referenced—long before their true natures were known.- Sources of misnomers...
for enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s due to their comparable effects on reaction rates and forms of catalysis involved.
Organocatalysts which display secondary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
functionality can be described as performing either enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
catalysis (by forming catalytic quantities of an active enamine nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
) or iminium
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
catalysis (by forming catalytic quantities of an activated iminium electrophile). This mechanism is typical for covalent organocatalysis. Covalent binding of substrate normally requires high catalyst loading (for proline-catalysis typically 20-30 mol%).
Noncovalent interactions such as hydrogen-bonding facilitates low catalyst loadings (down to 0.001 mol%).
Organocatalysis offers several advantages. There is no need for metal-based catalysis thus making a contribution to green chemistry
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
. In this context, simple organic acids have been used as catalyst for the modification of cellulose in water on multi-ton scale. When the organocatalyst is chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
an avenue is opened to asymmetric catalysis, for example the use of proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
in aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s,
Introduction
Regular achiral organocatalysts are based on nitrogen such as piperidinePiperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
used in the Knoevenagel condensation
Knoevenagel condensation
The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the Aldol condensation.A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule...
, DMAP
4-Dimethylaminopyridine
4-Dimethylaminopyridine is a derivative of pyridine with the chemical formula 2NC5H4N. This colourless solid is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich...
used in esterfications and DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
used in the Baylis-Hillman reaction. Thiazolium salts are employed in the Stetter reaction
Stetter reaction
The Stetter reaction is an organic reaction involving the nucleophile catalyzed conjugate addition of an aldehyde to a Michael acceptor such as an enone. The reaction product is a 1,4-dicarbonyl compound...
. These catalysts and reactions have a long history but current interest in organocatalysis is focused on asymmetric catalysis with chiral catalysts and this particular branch is called asymmetric organocatalysis or enantioselective organocatalysis . A pioneering reaction developed in the 1970s is called the Hajos-Parrish reaction
Hajos-Parrish-Eder-Sauer-Wiechert reaction
The Hajos–Parrish–Eder–Sauer–Wiechert reaction in organic chemistry is a proline catalysed asymmetric Aldol reaction. The reaction is named after its principal investigators from Hoffmann-La Roche and Schering AG...
:
In this reaction, naturally occurring chiral proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
is the chiral catalyst in an Aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
. The starting material is an achiral triketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
and it requires just 3% of proline to obtain the reaction product, a ketol in 93% enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
. This is the first example of an amino acid-catalyzed asymmetric aldol reaction.
The asymmetric synthesis of the Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
(1985) is also based on proline and another early application was one of the transformations in the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of Erythromycin
Erythromycin
Erythromycin is a macrolide antibiotic that has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often used for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma and...
by Robert B. Woodward (1981).
Many chiral organocatalysts are an adaptation of chiral ligand
Chiral ligand
In chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the...
s (which together with a metal center also catalyze asymmetric reactions) and both concepts overlap to some degree.
Organocatalyst classes
Organocatalysts for asymmetric synthesis can be grouped in several classes:- BiomoleculeBiomoleculeA biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
s: notably prolineProlineProline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
, phenylalaninePhenylalaninePhenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
. Secondary amines in general. The cinchona alkaloids, certain oligopeptideOligopeptideAn oligopeptide consists of between 2 and 20 amino acids and includes dipeptides, tripeptides, tetrapeptides, pentapeptides, etc.Examples of oligopeptides include:...
s. - Synthetic catalysts derived from biomolecules.
- Hydrogen bonding catalysts, including TADDOLS, derivatives of BINOL such as NOBINNOBINNOBIN is an organic molecule used for asymmetric catalysis. NOBIN is related to BINOL and other analogs by both having a chiral axis and being a scaffold for certain chemical reactions...
, and organocatalysts based on thioureaThioureaThiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
s - Triazolium salts as next-generation Stetter reactionStetter reactionThe Stetter reaction is an organic reaction involving the nucleophile catalyzed conjugate addition of an aldehyde to a Michael acceptor such as an enone. The reaction product is a 1,4-dicarbonyl compound...
catalysts
Examples of asymmetric reactions involving organocatalysts are:
- Asymmetric Diels-Alder reactions
- Asymmetric Michael reactions
- Asymmetric Mannich reactions
- Shi epoxidationShi epoxidationThe Shi epoxidation is a chemical reaction described as an asymmetric epoxidation of olefins with oxone and a fructose-derived catalyst ....
- Organocatalytic transfer hydrogenation
Imidazolidinone organocatalysis
A certain class of imidazolidinone compounds (also called MacMillan organocatalysts) are suitable catalysts for many asymmetric reactions such as asymmetric Diels-Alder reactions. The original such compound was derived from the biomoleculeBiomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
in two chemical steps (amidation with methylamine
Methylamine
Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
followed by condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
with acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
) which leave the chirality intact :
This catalyst works by forming a iminium ion with carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups of α,β-unsaturated aldehydes (enals) and enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s in a rapid chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
. This iminium activation is similar to activation of carbonyl groups by a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
and both catalysts lower the substrate's LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
:
The transient iminium intermediate is chiral which is transferred to the reaction product via chiral induction. The catalysts have been used in Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s, Michael additions, Friedel-Crafts alkylations, transfer hydrogenation
Transfer hydrogenation
Transfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
s and epoxidations.
One example is the asymmetric synthesis of the drug warfarin
Warfarin
Warfarin is an anticoagulant. It is most likely to be the drug popularly referred to as a "blood thinner," yet this is a misnomer, since it does not affect the thickness or viscosity of blood...
(in equilibrium with the hemiketal) in a Michael addition of 4-hydroxycoumarin and benzylideneacetone
Benzylideneacetone
Benzylideneacetone is the organic compound described by the formula C6H5CH=CHCCH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed...
:
A recent exploit is the vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
alkylation of crotonaldehyde
Crotonaldehyde
Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the E- and Z-isomers, which differ with respect to the relative position of the methyl and formyl groups. The E-isomer is more common...
with an organotrifluoroborate salt
Organotrifluoroborate
Organotrifluoroborates are organoboron compounds that contain an anion with the general formula [RBF3]−. They can be thought of as protected boronic acids, or as adducts of carbanions and boron trifluoride. Organotrifluoroborates are tolerant of air and moisture and are easy to handle and purify...
:
For other examples of its use: see organocatalytic transfer hydrogenation and asymmetric Diels-Alder reactions.
Thiourea organocatalysis
A large group of organocatalysts incorporate the ureaUrea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
or the thiourea
Thiourea
Thiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
moiety. These catalytically effective (thio)urea derivatives termed (thio)urea organocatalysts provide explicit double hydrogen-bonding interactions to coordinate and activate H-bond accepting substrates.