Oxygen isotope ratio cycle
Encyclopedia
Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass
of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite
in ocean core sample
s. The ratio is linked to water temperature of ancient oceans, which in turn reflects ancient climates. Cycles in the ratio mirror climate changes in geologic history.
(chemical symbol O) has three naturally occurring isotope
s: 16O, 17O
, and 18O
, where the 16, 17 and 18 refer to the atomic mass. The most abundant is 16O, with a small percentage of 18O and an even smaller percentage of 17O. Oxygen isotope
analysis considers only the ratio of 18O to 16O present in a sample.
The calculated ratio of the masses of each present in the sample is then compared to a standard, which can yield information about the temperature at which the sample was formed - see Proxy (climate)
for details.
s heavier than 16O and causes the water molecule in which it occurs to be heavier by that amount. The addition of more energy is required to vaporize H218O than H216O, and H218O liberates more energy when it condenses
. In addition, H216O tends to diffuse more rapidly.
Because H216O requires less energy to vaporize, and is more likely to diffuse to the liquid surface, the first water vapor formed during evaporation of liquid water is enriched in H216O, and the residual liquid is enriched in H218O. When water vapor condenses into liquid, H218O preferentially enters the liquid, while H216O is concentrated in the remaining vapor.
As an air mass moves from a warm region to a cold region, water vapor condenses and is removed as precipitation. The precipitation removes H218O, leaving progressively more H216O-rich water vapor. This distillation process causes precipitation to have lower 18O/16O as the temperature decreases. Additional factors can affect the efficiency of the distillation, such as the direct precipitation of ice crystals, rather than liquid water, at low temperatures.
Due to the intense precipitation that occurs in hurricanes, the H218O is exhausted relative to the H216O, resulting in relatively low 18O/16O ratios. The subsequent uptake of hurricane rainfall in trees, creates a record of the passing of hurricanes that can be used to create a historical record in the absence of human records.
s, or smaller cycles, superimposed on the large ones. This technique has been especially valuable for identifying glacial maxima and minima in the Pleistocene
.
, chemical formula CaCO3, is formed from water
, H2O, and carbon dioxide
, CO2, dissolved in the water. The carbon dioxide provides two of the oxygen atoms in the calcite. The calcium
must rob the third from the water. The isotope ratio in the calcite is therefore the same, after compensation, as the ratio in the water from which the microorganisms of a given layer extracted the material of the shell. The microorganism most frequently referenced is foraminifera
.
The sentence "The isotope ratio in the calcite is therefore the same, ..." is inexact: "proportionnal" would be more accurate (actually 1/3 of Oxygen present in calcite CaCO3; That is, without further chemistry details)
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite
Calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate . The other polymorphs are the minerals aragonite and vaterite. Aragonite will change to calcite at 380-470°C, and vaterite is even less stable.-Properties:...
in ocean core sample
Core sample
A core sample is a cylindrical section of a naturally occurring substance. Most core samples are obtained by drilling with special drills into the substance, for example sediment or rock, with a hollow steel tube called a core drill. The hole made for the core sample is called the "core hole". A...
s. The ratio is linked to water temperature of ancient oceans, which in turn reflects ancient climates. Cycles in the ratio mirror climate changes in geologic history.

Isotopes of oxygen
OxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(chemical symbol O) has three naturally occurring isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s: 16O, 17O
Oxygen-17
Oxygen-17 is a low abundant isotope of oxygen . Being the only stable isotope of oxygen possessing a nuclear spin and the unique characteristic of field-independent relaxation it enables NMR studies of metabolic pathways of compounds incorporating oxygen at high magnetic fields Oxygen-17 is a low...
, and 18O
Oxygen-18
Oxygen-18 is a natural, stable isotope of oxygen and one of the environmental isotopes.18O is an important precursor for the production of fluorodeoxyglucose used in positron emission tomography...
, where the 16, 17 and 18 refer to the atomic mass. The most abundant is 16O, with a small percentage of 18O and an even smaller percentage of 17O. Oxygen isotope
Isotopes of oxygen
There are three stable isotopes of oxygen that lead to oxygen having a standard atomic mass of 15.9994 u. 17 radioactive isotopes have also been characterized, with mass numbers from 12O to 28O, all short-lived, with the longest-lived being 15O with a half-life of 122.24 seconds...
analysis considers only the ratio of 18O to 16O present in a sample.
The calculated ratio of the masses of each present in the sample is then compared to a standard, which can yield information about the temperature at which the sample was formed - see Proxy (climate)
Proxy (climate)
In the study of past climates is known as paleoclimatology, climate proxies are preserved physical characteristics of the past that stand in for direct measurements , to enable scientists to reconstruct the climatic conditions that prevailed during much of the Earth's history...
for details.
Connection between isotopes and temperature/weather
18O is two neutronNeutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s heavier than 16O and causes the water molecule in which it occurs to be heavier by that amount. The addition of more energy is required to vaporize H218O than H216O, and H218O liberates more energy when it condenses
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
. In addition, H216O tends to diffuse more rapidly.
Because H216O requires less energy to vaporize, and is more likely to diffuse to the liquid surface, the first water vapor formed during evaporation of liquid water is enriched in H216O, and the residual liquid is enriched in H218O. When water vapor condenses into liquid, H218O preferentially enters the liquid, while H216O is concentrated in the remaining vapor.
As an air mass moves from a warm region to a cold region, water vapor condenses and is removed as precipitation. The precipitation removes H218O, leaving progressively more H216O-rich water vapor. This distillation process causes precipitation to have lower 18O/16O as the temperature decreases. Additional factors can affect the efficiency of the distillation, such as the direct precipitation of ice crystals, rather than liquid water, at low temperatures.
Due to the intense precipitation that occurs in hurricanes, the H218O is exhausted relative to the H216O, resulting in relatively low 18O/16O ratios. The subsequent uptake of hurricane rainfall in trees, creates a record of the passing of hurricanes that can be used to create a historical record in the absence of human records.
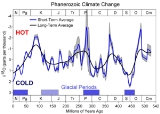
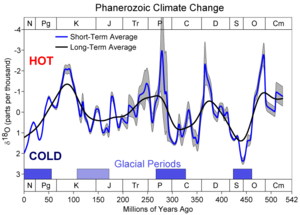
Connection between temperature and climate
The 18O/16O ratio provides a record of ancient water temperature. Water 10 to 15 degrees Celsius (18 to 27 degrees Fahrenheit) cooler than present represents glaciation. Precipitation and therefore glacial ice contain water with a low 18O content. Since large amounts of 16O water are being stored as glacial ice, the 18O content of oceanic water is high. Water up to 5 degrees Celsius (9 °F) warmer than today represents an interglacial, when the 18O content of oceanic water is lower. A plot of ancient water temperature over time indicates that climate has varied cyclically, with large cycles and harmonicHarmonic
A harmonic of a wave is a component frequency of the signal that is an integer multiple of the fundamental frequency, i.e. if the fundamental frequency is f, the harmonics have frequencies 2f, 3f, 4f, . . . etc. The harmonics have the property that they are all periodic at the fundamental...
s, or smaller cycles, superimposed on the large ones. This technique has been especially valuable for identifying glacial maxima and minima in the Pleistocene
Pleistocene
The Pleistocene is the epoch from 2,588,000 to 11,700 years BP that spans the world's recent period of repeated glaciations. The name pleistocene is derived from the Greek and ....
.
Connection between calcite and water
Limestone is deposited from the calcite shells of microorganisms. Calcite, or calcium carbonateCalcium carbonate
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
, chemical formula CaCO3, is formed from water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, H2O, and carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, CO2, dissolved in the water. The carbon dioxide provides two of the oxygen atoms in the calcite. The calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
must rob the third from the water. The isotope ratio in the calcite is therefore the same, after compensation, as the ratio in the water from which the microorganisms of a given layer extracted the material of the shell. The microorganism most frequently referenced is foraminifera
Foraminifera
The Foraminifera , or forams for short, are a large group of amoeboid protists which are among the commonest plankton species. They have reticulating pseudopods, fine strands of cytoplasm that branch and merge to form a dynamic net...
.
The sentence "The isotope ratio in the calcite is therefore the same, ..." is inexact: "proportionnal" would be more accurate (actually 1/3 of Oxygen present in calcite CaCO3; That is, without further chemistry details)

