PKC alpha
Encyclopedia
Protein kinase C alpha also known as PRKCA, refers to both a human gene
and the protein
that is encoded by it.
family. These enzymes are characterized by their ability to add a phosphate group to other proteins, thus changing their function. PKC-α has been widely studied in the tissues of many organisms including drosophila, xenopus, cow, dog, chicken, human, monkey, mouse, pig, and rabbit. Many studies are currently being conducted investigating the structure, function, and regulation of this enzyme. The most recent investigations concerning this enzyme include its general regulation, hepatic function, and cardiac function.
family is regulated by allosteric regulation, the binding of a modulating molecule that effects a conformational change in the enzyme and thus a change in the enzyme’s activity. The primary mode of PKC-α’s regulation, however, involves its interaction with the cell membrane, not direct interaction with specific molecules. The cell membrane consists of phospholipids. At warmer temperatures, phospholipids exist in a more fluid state as a result of increased intramolecular motion. The more fluid the cell membrane, the greater PKC-α’s activity. At cooler temperatures, phospholipids are found in a solid state with constricted motion. As phospholipids become stationary, they assume a particular orientation within the membrane. Phospholipids that solidify at an irregular or angled orientation with respect to the membrane, can reduce PKC-α’s activity.
The composition of the cell membrane can also affect PKC-α’s function. The presence of calcium ions, magnesium ions, and diacylglycerols (DAGs) are the most important because they influence the hydrophobic domain of the membrane. Varying concentrations of these three components constitute a longer or shorter length of the hydrophobic domain. Membranes with long hydrophobic domains result in decreased activity because it is harder for PKC-α to insert into the membrane. At low concentrations, the hydrophobic domain is shorter allowing PKC-α to readily insert into the membrane and its activity increases.
techniques, researchers have demonstrated that the secondary structure of PKC alpha consists of around 44% beta sheets and nearly 22% alpha helices at 20°C. Upon addition of calcium ions, a slight increase in beta sheets to 48% was observed. Additional ligands normally associated with PKC alpha, such as PMA, ATP, and phospholipids had no effect on secondary structure.
The structure of PKC alpha was better preserved during denaturation of the enzyme at 75°C in the presence of calcium ions than in their absence. In one study, beta sheet
composition only decreased by 13% with calcium ions present compared to 19% when absent.
growth factors are able to enter the cell and increase the rate of cell growth. This is thought to be a promotional event that may prolong certain epithelial cancers.
, which results from a lack of blood supply to the myocardium (heart muscle tissue). Recent research into the role of PKC alpha in cardiac tissue has indicated that it has an important role in stimulating hypertrophy
. This was demonstrated by the ability of agonist-mediated hypertrophy
to be stopped only as a result of the inhibition of PKC alpha in an experiment in situ. However, in further in vivo
research using mice, the transgenic overexpression of PKC alpha showed no effect on cardiac growth, and the inhibition of PKC alpha showed no effect on hypertrophic response to increased cardiac pressure. On the contrary, research has shown that removing PKC alpha altogether improved the hearts ability to contract.
In summary, research is pointing in the direction that PKC alpha’s role in cardiac tissue has more impact as a regulator of contractility than of hypertrophy
. In another study, the binding peptides, RACK and others derived from PKC beta, were expressed in mouse hearts. The genetic code for these proteins are similar to those of all isoforms of the PKC family (alpha, beta, and gamma). As such, RACK and other proteins can regulate the expression of all PKC family proteins. In this particular study, however, only PKC alpha was affected. Again, overexpression caused decreased contractile performance, whereas inhibition saw increased performance.
. Phospholipase D is located on the plasma membrane and is responsible for hydrolyzing phosphatidylcholine to phosphatidic acid and choline
. Research has indicated that phospholipase D may play roles in tumorigenesis by altering cellular events such as invasion and migration. Point mutations at particular phenylalanine
residues have shown to inhibit PKC-α’s ability to activate phospholipase D. Current research is being conducted investigating PKC-α’s inhibitory affects. Researchers hope to learn how to exploit PKC-α’s ability to turn down phospholipase D’s activity and use this function to create anti-cancer drugs.
Another breakthrough branch of research concerning PKC-α concerns its role in erythrocyte (red blood cell) development. Currently, researchers understand that PKC-α is correlated with the differentiation of erythroid progenitor cells in bone marrow. These undifferentiated cells give rise to the mass of red blood cells present in blood. Future research endeavors seek to find whether it is activation or inhibition of PKC-α which affects the development of erythrocytes. By answering this question, scientists hope to gain insight into various types of hematologic diseases such as aplastic anemia and leukemia.
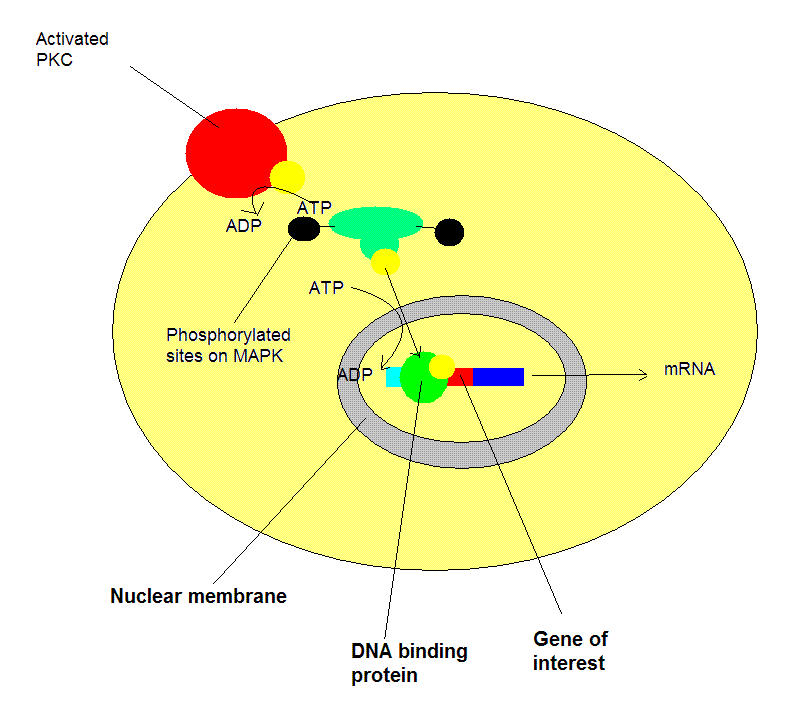
with FSCN1
, Epidermal growth factor receptor
, C1QBP
, Oxoguanine glycosylase
and CD29
.
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
and the protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
that is encoded by it.
Background
Protein kinase C-alpha (PKC-α) is a specific member of the protein kinaseProtein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
family. These enzymes are characterized by their ability to add a phosphate group to other proteins, thus changing their function. PKC-α has been widely studied in the tissues of many organisms including drosophila, xenopus, cow, dog, chicken, human, monkey, mouse, pig, and rabbit. Many studies are currently being conducted investigating the structure, function, and regulation of this enzyme. The most recent investigations concerning this enzyme include its general regulation, hepatic function, and cardiac function.
Regulation
PKC-α is unique in its mode of regulation compared to other kinases within this family. In general, the protein kinaseProtein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
family is regulated by allosteric regulation, the binding of a modulating molecule that effects a conformational change in the enzyme and thus a change in the enzyme’s activity. The primary mode of PKC-α’s regulation, however, involves its interaction with the cell membrane, not direct interaction with specific molecules. The cell membrane consists of phospholipids. At warmer temperatures, phospholipids exist in a more fluid state as a result of increased intramolecular motion. The more fluid the cell membrane, the greater PKC-α’s activity. At cooler temperatures, phospholipids are found in a solid state with constricted motion. As phospholipids become stationary, they assume a particular orientation within the membrane. Phospholipids that solidify at an irregular or angled orientation with respect to the membrane, can reduce PKC-α’s activity.
The composition of the cell membrane can also affect PKC-α’s function. The presence of calcium ions, magnesium ions, and diacylglycerols (DAGs) are the most important because they influence the hydrophobic domain of the membrane. Varying concentrations of these three components constitute a longer or shorter length of the hydrophobic domain. Membranes with long hydrophobic domains result in decreased activity because it is harder for PKC-α to insert into the membrane. At low concentrations, the hydrophobic domain is shorter allowing PKC-α to readily insert into the membrane and its activity increases.
Secondary Protein Structure Determination
Using infrared spectroscopyInfrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
techniques, researchers have demonstrated that the secondary structure of PKC alpha consists of around 44% beta sheets and nearly 22% alpha helices at 20°C. Upon addition of calcium ions, a slight increase in beta sheets to 48% was observed. Additional ligands normally associated with PKC alpha, such as PMA, ATP, and phospholipids had no effect on secondary structure.
The structure of PKC alpha was better preserved during denaturation of the enzyme at 75°C in the presence of calcium ions than in their absence. In one study, beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
composition only decreased by 13% with calcium ions present compared to 19% when absent.
Epithelial Studies
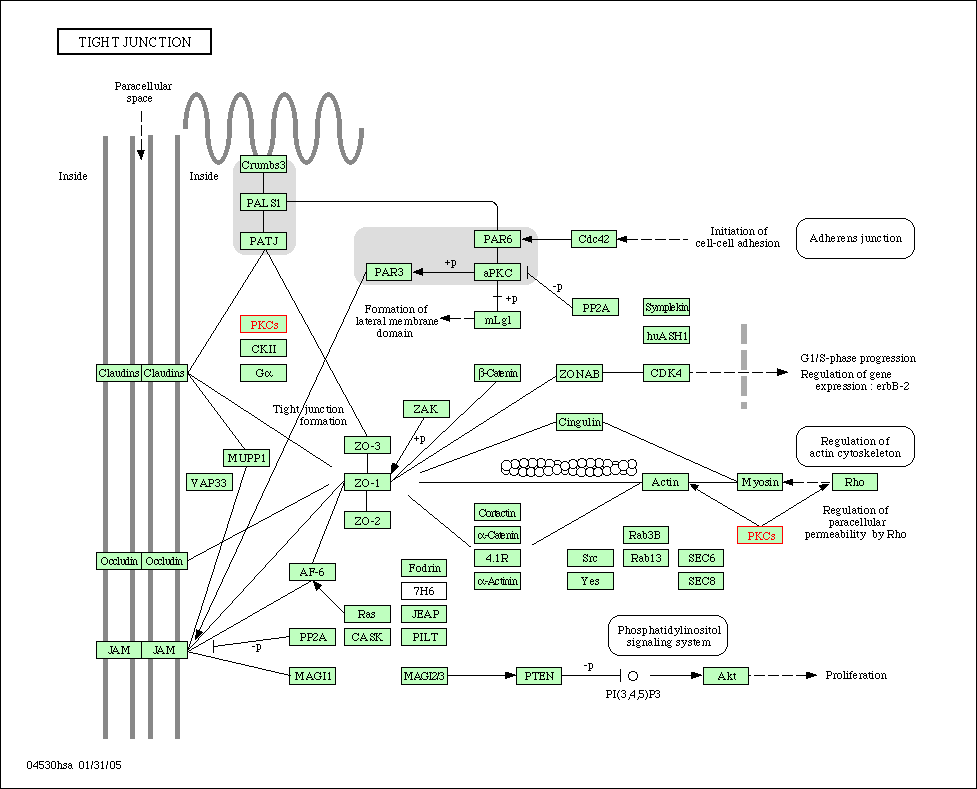
Another field of research has indicated that PKC-α plays a vital role in epithelial tissue, the tissue that covers all external and internal surfaces of the body. Specifically, PKC-α is involved in altering the function of tight junctions. Tight junctions exist at the meeting point between two cells. Here, tight junctions fuse together to form an impermeable barrier to not only large molecules such as proteins, but also smaller molecules like water. This prevents foreign molecules from entering the cell and helps regulate the internal environment of the cell. Cells infected with certain types of epithelial cancer exhibit increased PKC-α activity. This is a result of a change in the shape of the cell membrane, particularly in the areas where tight junctions exists. With greater activity of PKC-α, the tight junctions lose their ability to form a tight barrier. This causes an increased leakiness of the tight junctions and thus movement of molecules into the cells. In intestinal areas, luminalLuminal
Luminal may refer to:* A trade name for the anti-epileptic drug phenobarbital* Luminal , a 2004 film by Italian director Andrea Vecchiato starring Denis Lavant* In biology, pertaining to the lumen, the interior of a hollow structure...
growth factors are able to enter the cell and increase the rate of cell growth. This is thought to be a promotional event that may prolong certain epithelial cancers.

Hepatic Studies
Much of the research of PKC alpha pertaining to its role in liver tissue involves the effects of bile acids on the phosphorylation mechanism of the PKC family of proteins. Past research has affirmed that the bile acid CDCA inhibits the healthy glucagon response through a phosphorylation-related sequence. In related studies further testing the effects of CDCA on hepatocytes, CDCA was shown to have induced PKC translocation to the plasma membrane. PKC alpha was favored in this process over PKC delta. The implications of this finding are that increased interaction between the glucagon receptor and PKC alpha could occur.Cardiac Studies
PKC alpha is one of the lesser studied proteins of the PKC family because it is not highly regulated in the serious medical condition known as acute myocardial ischemiaIschemia
In medicine, ischemia is a restriction in blood supply, generally due to factors in the blood vessels, with resultant damage or dysfunction of tissue. It may also be spelled ischaemia or ischæmia...
, which results from a lack of blood supply to the myocardium (heart muscle tissue). Recent research into the role of PKC alpha in cardiac tissue has indicated that it has an important role in stimulating hypertrophy
Hypertrophy
Hypertrophy is the increase in the volume of an organ or tissue due to the enlargement of its component cells. It should be distinguished from hyperplasia, in which the cells remain approximately the same size but increase in number...
. This was demonstrated by the ability of agonist-mediated hypertrophy
Hypertrophy
Hypertrophy is the increase in the volume of an organ or tissue due to the enlargement of its component cells. It should be distinguished from hyperplasia, in which the cells remain approximately the same size but increase in number...
to be stopped only as a result of the inhibition of PKC alpha in an experiment in situ. However, in further in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
research using mice, the transgenic overexpression of PKC alpha showed no effect on cardiac growth, and the inhibition of PKC alpha showed no effect on hypertrophic response to increased cardiac pressure. On the contrary, research has shown that removing PKC alpha altogether improved the hearts ability to contract.
In summary, research is pointing in the direction that PKC alpha’s role in cardiac tissue has more impact as a regulator of contractility than of hypertrophy
Hypertrophy
Hypertrophy is the increase in the volume of an organ or tissue due to the enlargement of its component cells. It should be distinguished from hyperplasia, in which the cells remain approximately the same size but increase in number...
. In another study, the binding peptides, RACK and others derived from PKC beta, were expressed in mouse hearts. The genetic code for these proteins are similar to those of all isoforms of the PKC family (alpha, beta, and gamma). As such, RACK and other proteins can regulate the expression of all PKC family proteins. In this particular study, however, only PKC alpha was affected. Again, overexpression caused decreased contractile performance, whereas inhibition saw increased performance.
Future Research Prospects
PKC-α shows important regulation of phospholipase DPhospholipase D
Phospholipase D is an enzyme which is located in the plasma membrane and catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid , releasing the soluble choline headgroup into the cytosol...
. Phospholipase D is located on the plasma membrane and is responsible for hydrolyzing phosphatidylcholine to phosphatidic acid and choline
Choline
Choline is a water-soluble essential nutrient. It is usually grouped within the B-complex vitamins. Choline generally refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation....
. Research has indicated that phospholipase D may play roles in tumorigenesis by altering cellular events such as invasion and migration. Point mutations at particular phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
residues have shown to inhibit PKC-α’s ability to activate phospholipase D. Current research is being conducted investigating PKC-α’s inhibitory affects. Researchers hope to learn how to exploit PKC-α’s ability to turn down phospholipase D’s activity and use this function to create anti-cancer drugs.
Another breakthrough branch of research concerning PKC-α concerns its role in erythrocyte (red blood cell) development. Currently, researchers understand that PKC-α is correlated with the differentiation of erythroid progenitor cells in bone marrow. These undifferentiated cells give rise to the mass of red blood cells present in blood. Future research endeavors seek to find whether it is activation or inhibition of PKC-α which affects the development of erythrocytes. By answering this question, scientists hope to gain insight into various types of hematologic diseases such as aplastic anemia and leukemia.

Genes Associated with PKC alpha
PICK1Interactions
PKC alpha has been shown to interactProtein-protein interaction
Protein–protein interactions occur when two or more proteins bind together, often to carry out their biological function. Many of the most important molecular processes in the cell such as DNA replication are carried out by large molecular machines that are built from a large number of protein...
with FSCN1
FSCN1
Fascin is a protein that in humans is encoded by the FSCN1 gene.-Interactions:FSCN1 has been shown to interact with Low affinity nerve growth factor receptor and PKC alpha.-Further reading:...
, Epidermal growth factor receptor
Epidermal growth factor receptor
The epidermal growth factor receptor is the cell-surface receptor for members of the epidermal growth factor family of extracellular protein ligands...
, C1QBP
C1QBP
Complement component 1 Q subcomponent-binding protein, mitochondrial is a protein that in humans is encoded by the C1QBP gene.-Interactions:C1QBP has been shown to interact with Protein kinase D1, BAT2, PRKCD, PKC alpha and Protein kinase Mζ....
, Oxoguanine glycosylase
Oxoguanine glycosylase
8-Oxoguanine glycosylase also known as OGG1 is a DNA glycosylase enzyme that, in humans, is encoded by the OGG1 gene. It is involved in base excision repair.- Function :...
and CD29
CD29
Integrin beta-1 is a protein that in humans is encoded by the ITGB1 gene. CD29 is an integrin unit associated with very late antigen receptors. It is known to conjoin with alpha-3 subunit to create α3β1 complex that reacts to such molecules as netrin-1 and reelin.Integrins are heterodimeric...
.

