
Paclitaxel
Encyclopedia
Paclitaxel is a mitotic inhibitor
used in cancer
chemotherapy
. It was discovered in a U.S. National Cancer Institute
program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani
isolated it from the bark of the Pacific yew tree, Taxus brevifolia
and named it taxol. When it was developed commercially by Bristol-Myers Squibb
(BMS) the generic name was changed to paclitaxel and the BMS compound is sold under the trademark
TAXOL. In this formulation, paclitaxel is dissolved in Cremophor EL
and ethanol
, as a delivery agent. A newer formulation, in which paclitaxel is bound to albumin
, is sold under the trademark Abraxane.
Paclitaxel is now used to treat patients with lung
, ovarian
, breast cancer
, head and neck cancer, and advanced forms of Kaposi's sarcoma
. Paclitaxel is also used for the prevention of restenosis.
Paclitaxel stabilizes microtubules and as a result, interferes with the normal breakdown of microtubules during cell division. Together with docetaxel, it forms the drug category of the taxane
s. It was the subject of a notable total synthesis by Robert A. Holton
.
While offering substantial improvement in patient care, paclitaxel has been a relatively controversial drug. There was originally concern because of the environmental impact of its original sourcing, no longer used, from the Pacific yew. In addition, the assignment of rights, and even the name itself, to Bristol-Myers Squibb were the subject of public debate and Congressional hearings.
) skeleton. There are a total of 11 stereocenters. The active stereoisomer is (-)-paclitaxel (shown here).
(NCI) in the United States set up the Cancer Chemotherapy National Service Center (CCNSC) to act as a public screening center for anticancer activity in compounds submitted by external institutions and companies. Although the majority of compounds screened were of synthetic origin, one chemist, Jonathan Hartwell, who was employed there from 1958 onwards, had had experience with natural product
derived compounds, and began a plant screening operation. After some years of informal arrangements, in July 1960, the NCI commissioned USDA botanists to collect samples from about 1000 plant species per year. On 21 August 1962, one of those botanists, Arthur S. Barclay, collected bark from a single Pacific yew tree, Taxus brevifolia
, in a forest north of the town of Packwood, Washington
as part of a four month trip to collect material from over 200 different species. The material was then processed by a number of specialist CCNSC subcontractors, and one of the Taxus samples was found to be cytotoxic in a cellular assay on 22 May 1964.
Accordingly, in late 1964 or early 1965, the fractionation and isolation laboratory run by Monroe E. Wall in Research Triangle Park
, North Carolina
, began work on fresh Taxus samples, isolating the active ingredient in September 1966 and announcing their findings at an April 1967 American Chemical Society
meeting in Miami Beach. They named the pure compound taxol in June 1967.
Wall and his colleague Wani published their results, including the chemical structure, in 1971.
The NCI continued to commission work to collect more Taxus bark and to isolate increasing quantities of taxol. By 1969, 28 kg of crude extract had been isolated from almost 1,200 kg of bark, although this ultimately yielded only 10g of pure material, but for several years, no use was made of the compound by the NCI. In 1975, it was shown to be active in another in vitro system ; two years later a new department head reviewed the data and finally recommended taxol be moved on to the next stage in the discovery process. This required increasing quantities of purified taxol, up to 600g, and in 1977 a further request for 7,000 lbs of bark was made.
In 1978, two NCI researchers published a report showing taxol was mildly effective in leukaemic mice.
In November 1978, taxol was shown to be effective in xenograft studies.
Meanwhile, taxol began to be well known in the cell biology, as well as the cancer community, with a publication in early 1979 by Susan B. Horwitz, a molecular pharmacologist at Albert Einstein College of Medicine
, showing taxol had a previously unknown mechanism of action involving the stabilization of microtubules. Together with formulation problems, this increased interest from researchers meant that by 1980, the NCI envisaged needing to collect 20,000 lbs of bark.
Animal toxicology studies were complete by June 1982, and in November NCI applied for the IND
necessary to begin clinical trials in humans.
trials was made a year later. These larger trials needed more bark and collection of a further 12,000 pounds was commissioned, which enabled some phase II trials to begin by the end of 1986. But by then it was recognized that the demand for taxol might be substantial and that more than 60,000 pounds of bark might be needed as a minimum. This unprecedentedly large amount brought ecological concerns about the impact on yew populations into focus for the first time, as local politicians and foresters expressed unease at the program.
The first public report from a phase II trial in May 1988 showed an effect in melanoma patients and a remarkable response rate of 30% in patients with refractory ovarian cancer.
At this point, Gordon Cragg of the NCI's Natural Product Branch calculated the synthesis of enough taxol to treat all the ovarian cancer and melanoma cases in the US would require the destruction of 360,000 trees annually. For the first time, serious consideration was given to the problem of supply.
Because of the practical and, in particular, the financial scale of the program needed, the NCI decided to seek association with a pharmaceutical company, and in August 1989, it published a Cooperative Research and Development Agreement
(CRADA) offering its current stock and supply from current bark stocks, and proprietary access to the data so far collected, to a company willing to commit to providing the funds to collect further raw material, isolate taxol, and fund a large proportion of clinical trials. In the words of Goodman and Welsh, authors of a substantial scholarly book on taxol,
Although the offer was widely advertised, only four companies responded to the CRADA, including the American firm Bristol-Myers Squibb
(BMS),
which was selected as the partner in December 1989. The choice of BMS later became controversial and was the subject of Congressional hearings in 1991 and 1992. While it seems clear the NCI had little choice but to seek a commercial partner, there was also controversy about the terms of the deal, eventually leading to a report by the General Accounting Office in 2003, which concluded the NIH had failed to ensure value for money. In related CRADAs with the USDA and Department of the Interior, Bristol-Myers Squibb was given exclusive first refusal on all Federal supplies of Taxus brevifolia.
This exclusive contract lead to some criticism for giving BMS a "cancer monopoly
".
Eighteen months after the CRADA, BMS filed a new drug application
(NDA), which was given FDA approval at the very end of 1992.
Although there was no patent on the compound, the provisions of the Waxman-Hatch Act gave Bristol-Myers Squibb five years exclusive marketing rights.
In 1990, BMS applied to trademark the name taxol as TAXOL. This was controversially approved in 1992. At the same time, paclitaxel replaced taxol as the generic name of the compound. Critics, including the journal Nature
, argued the name taxol had been used for more than two decades and in more than 600 scientific articles and suggested the trademark should not have been awarded and the BMS should renounce its rights to it. BMS argued changing the name would cause confusion among oncologists and possibly endanger the health of patients. BMS has continued to defend its rights to the name in the courts.
BMS has also been criticized for misrepresentation by Goodman and Walsh, who quote from a company report saying
This quote is, strictly speaking, accurate: the objection seems to be that this misleadingly neglects to explain that it was the scientist doing the isolation who named the compound taxol and it was not referred to in any other way for more than twenty years.
Annual sales peaked in 2000, reaching US$1.6 billion; paclitaxel is now available in generic form.

 From 1967 to 1993, almost all paclitaxel produced was derived from bark from the Pacific yew, the harvesting of which kills the tree in the process. The processes used were descendants of the original isolation method of Wall and Wani; by 1987, the NCI had contracted Hauser Chemical Research of Boulder, Colorado, to handle bark on the scale needed for Phase II and III trials. While there was considerable uncertainty about how large the wild population of Taxus brevifola was and what the eventual demand for taxol would be, it had been clear for many years that an alternative, sustainable source of supply would be needed. Initial attempts used needles from the tree, or material from other related Taxus species, including cultivated ones, but these attempts were bedevilled by the relatively low and often highly variable yields obtained. It was not until the early 1990s, at a time of increased sensitivity to the ecology of the forests of the Pacific Northwest
From 1967 to 1993, almost all paclitaxel produced was derived from bark from the Pacific yew, the harvesting of which kills the tree in the process. The processes used were descendants of the original isolation method of Wall and Wani; by 1987, the NCI had contracted Hauser Chemical Research of Boulder, Colorado, to handle bark on the scale needed for Phase II and III trials. While there was considerable uncertainty about how large the wild population of Taxus brevifola was and what the eventual demand for taxol would be, it had been clear for many years that an alternative, sustainable source of supply would be needed. Initial attempts used needles from the tree, or material from other related Taxus species, including cultivated ones, but these attempts were bedevilled by the relatively low and often highly variable yields obtained. It was not until the early 1990s, at a time of increased sensitivity to the ecology of the forests of the Pacific Northwest
, that taxol was successfully extracted on a clinically useful scale from these sources.
From the late 1970s, chemists in the US and France had been interested in taxol. A number of US groups, including one led by Robert A. Holton
, attempted a total synthesis of the molecule, starting from petrochemical
-derived starting materials. This work was primarily motivated as a way of generating chemical knowledge, rather than with any expectation of developing a practical production technique. By contrast, the French group of Pierre Potier at the CNRS quickly recognized the problem of yield. His laboratory was on a campus populated by the related yew Taxus baccata, so needles were available locally in large quantity. By 1981, he had shown that it was feasible to isolate relatively large quantities of the compound 10-deacetylbaccatin
, a plausible first step for a semisynthetic production route to taxol. By 1988 he copublished such a semisynthetic route from needles of T. baccata.
The view of the NCI, however, was even this route was not practical.
By 1988, and particularly with Potier's publication, it was clear to Holton as well a practical semisynthetic production route would be important. By late 1989, Holton's group had developed a semisynthetic route to paclitaxel with twice the yield of the Potier process. Florida State University
, where Holton worked, signed a deal with Bristol-Myers Squibb
to license this and future patents. In 1992, Holton patented an improved process with an 80% yield. BMS took the process in-house and started to manufacture paclitaxel in Ireland from 10-deacetylbaccatin isolated from the needles of the European yew. In early 1993, BMS was able to announce that it would cease reliance on Pacific yew bark by the end of 1995, effectively terminating the ecological controversy over its use. This announcement also made good their commitment to develop an alternative supply route, made to the NCI in their CRADA application of 1989.
Currently, all paclitaxel production for BMS uses plant cell fermentation (PCF) technology developed by the German and Canadian biotechnology company Phyton Biotech, Inc and carried out at their plant in Germany. This starts from a specific Taxus cell line propagated in aqueous medium in large fermentation tanks. Paclitaxel is then extracted directly, purified by chromatography and isolated by crystallization. Compared to the semisynthesis, PCF eliminates the need for many hazardous chemicals and saves a considerable amount of energy.
In 1993, taxol was coincidentally discovered to be produced in a newly described fungus
living in the yew tree. It has since been found in a number of other endophytic
fungi, including Nodulisporium sylviforme, Alternaria taxi, Cladosporium cladosporioides MD2, Metarhizium anisopliae, Aspergillus candidus MD3, Mucor rouxianus sp., Chaetomella raphigera, Phyllosticta tabernaemontanae, Phomopsis, Pestalotiopsis pauciseta, Phyllosticta citricarpa, Podocarpus,Fusarium solani, Pestalotiopsis terminaliae, Pestalotiopsis breviseta, Botryodiplodia theobromae Pat., Gliocladium sp., Alternaria alternata var. monosporus, Cladosporium cladosporioides, Nigrospora sp., Pestalotiopsis versicolor, and Taxomyces andreanae, opening the possibility of taxol production by culturing one of these fungal species.
The initial motivation for synthetic approaches to paclitaxel included the opportunity to create closely related compounds. Indeed, this approach led to the development of docetaxel.
s in the shells and leaves of hazel plants, including paclitaxel
, 10-deacetylbaccatin III, baccatin III, paclitaxel C, and 7-epipaclitaxel. The finding of these compounds in shells, which are considered discarded material and are mass produced by many food industries, is of interest for the future availability of paclitaxel (Taxol).
that inhibit microtubule assembly, paclitaxel stabilizes the microtubule
polymer and protects it from disassembly. Chromosomes are thus unable to achieve a metaphase spindle configuration. This blocks progression of mitosis, and prolonged activation of the mitotic checkpoint triggers apoptosis or reversion to the G-phase of the cell cycle without cell division,.
The ability of paclitaxel to inhibit spindle function is generally attributed to its suppression of microtubule dynamics, but recent studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher therapeutic concentrations, paclitaxel appears to suppress microtubule detachment from centrosomes, a process normally activated during mitosis. The binding site for paclitaxel has been identified on the beta-tubulin subunit.
.
It is recommended in NICE
guidance of June 2001 that it should be used for nonsmall cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should not be used in the adjuvant treatment of early node-positive breast cancer.
The cost to the NHS per patient in early breast cancer, assuming four cycles of treatment, is about £4000 (approx. $6000).
-bound paclitaxel (trade name Abraxane, also called nab-paclitaxel) is an alternative formulation where paclitaxel is bound to albumin nano-particles.
Much of the clinical toxicity of the original paclitaxel formulation is associated with the solvent Cremophor EL
in which it is dissolved for delivery.
Much of the clinical toxicity of paclitaxel is associated with the solvent Cremophor EL
in which it is dissolved for delivery.
Abraxis BioScience
developed Abraxane, in which paclitaxel is bonded to albumin
as an alternative delivery agent to the often toxic solvent delivery method. This was approved by the U.S. Food and Drug Administration
in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy.
The closely related taxane docetaxel has a similar set of clinical uses to paclitaxel. It is marketed under the name of Taxotere.
(recurrent narrowing) of coronary stent
s; locally delivered to the wall of the coronary artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents. Paclitaxel drug eluting coated stents
are sold under the trade name Taxus by Boston Scientific
in the United States.
by ovarian damage and chest pain can also occur. A number of these side effects are associated with the excipient
used, Cremophor EL, a polyoxyethylated castor oil
. Allergies to drugs such as cyclosporine, teniposide and drugs containing polyoxyethylated castor oil may indicate increased risk of adverse reactions to paclitaxel. Dexamethasone
is given prior to beginning paclitaxel treatment to mitigate some of the side effects. Leuprolide
, a GnRH analog
may prevent ovarian damage, according to mice studies.
Protarga has linked paclitaxel to docosahexaenoic acid
(DHA), a fatty acid easily taken up by tumor cells; the DHA-paclitaxel
“appears not to be cytotoxic until the bond with DHA is cleaved within the cell.” The advantage of DHA-paclitaxel over paclitaxel is DHA-paclitaxel’s ability to carry much higher concentrations of paclitaxel to the cells, which are maintained for longer periods in the tumor cells, thus increasing their action. With increased activity, DHA-paclitaxel, also known as Taxoprexin, may have a more successful response in cancer patients than paclitaxel, and it may be able to treat more types of cancer than paclitaxel has been able to treat.
Cell Therapeutics has formulated PG-paclitaxel, which is paclitaxel bonded to a polyglutamate polymer; tumor cells are significantly more porous to polyglutamate polymers than normal cells, due to the leaky endothelial membranes of tumor cells. PG-paclitaxel has been introduced into clinical use, and has proven to initiate very mild side effects and to effectively treat many patients who were not responsive to the action of Taxol. The PG-paclitaxel may be a very promising anticancer drug, as it is much more selective than paclitaxel for which cells it targets.
ImmunoGen has been introducing tumor-activated prodrug (TAP) technology in recent years, and is now working to apply this technology to paclitaxel. Tumor-activated Taxol prodrugs are designed for accurate targeting, by the action of a monoclonal antibody which is very specific to certain cells. Tumor-activated Taxol prodrugs research is progressing, and in mice, the “taxane-based TAP completely eradicated human tumour xenografts at non-toxic doses.”
ANG1005
is made up of one molecule of a peptide called angiopep-2 joined with three molecules of paclitaxel. It is in phase I clinical trials for some types of cancer.
stabilizer. In vitro
assays involving microtubules, such as motility assays, generally rely on paclitaxel to maintain microtubule integrity in the absence of the various nucleating factors and other stabilizing elements found in the cell. For example, it is used for in vitro tests of drugs that aim to alter the behavior of microtubule motor proteins, or for studies of mutant motor proteins. Paclitaxel is sometimes used for in vivo
studies as well; it can be fed to test organisms, such as fruit flies
, or injected into individual cells, to inhibit microtubule disassembly or to increase the number of microtubules in the cell. Paclitaxel induces remyelination in a demyelinating mouse in vivo and inhibits hPAD2 in vitro though its methyl ester side chain did not. Angiotech Pharmaceuticals Inc. began phase II clinical trials in 1999 as a multiple sclerosis treatment but in 2002, reported that the results showed no statistical significance.
pathway, parts of which have been successfully transplanted into production strains of E.coli and yeast
Mitotic inhibitor
A mitotic inhibitor is a drug that inhibits mitosis, or cell division. These drugs disrupt microtubules, which are structures that pull the cell apart when it divides...
used in cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
chemotherapy
Chemotherapy
Chemotherapy is the treatment of cancer with an antineoplastic drug or with a combination of such drugs into a standardized treatment regimen....
. It was discovered in a U.S. National Cancer Institute
National Cancer Institute
The National Cancer Institute is part of the National Institutes of Health , which is one of 11 agencies that are part of the U.S. Department of Health and Human Services. The NCI coordinates the U.S...
program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani
Mansukh C. Wani
Professor Mansukh C. Wani, Ph.D. is a principal scientist at the Research Triangle Institute in North Carolina. He is co-discoverer of Taxol and camptothecin, two anti-cancer drugs considered standard in the treatment to fight ovarian, breast, lung and colon cancers. In 2000, Dr. Wani received an...
isolated it from the bark of the Pacific yew tree, Taxus brevifolia
Taxus brevifolia
Taxus brevifolia is a conifer native to the Pacific Northwest of North America. It ranges from southernmost Alaska south to central California, mostly in the Pacific Coast Ranges, but with an isolated disjunct population in southeast British Columbia, most notably occurring on Zuckerberg Island...
and named it taxol. When it was developed commercially by Bristol-Myers Squibb
Bristol-Myers Squibb
Bristol-Myers Squibb , often referred to as BMS, is a pharmaceutical company, headquartered in New York City. The company was formed in 1989, following the merger of its predecessors Bristol-Myers and the Squibb Corporation...
(BMS) the generic name was changed to paclitaxel and the BMS compound is sold under the trademark
Trademark
A trademark, trade mark, or trade-mark is a distinctive sign or indicator used by an individual, business organization, or other legal entity to identify that the products or services to consumers with which the trademark appears originate from a unique source, and to distinguish its products or...
TAXOL. In this formulation, paclitaxel is dissolved in Cremophor EL
Cremophor EL
Cremophor EL is the registered trademark of BASF Corp. for its version of polyethoxylated castor oil. It is prepared by reacting 35 moles of ethylene oxide with each mole of castor oil...
and ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, as a delivery agent. A newer formulation, in which paclitaxel is bound to albumin
Human serum albumin
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin constitutes about half of the blood serum protein...
, is sold under the trademark Abraxane.
Paclitaxel is now used to treat patients with lung
Lung cancer
Lung cancer is a disease characterized by uncontrolled cell growth in tissues of the lung. If left untreated, this growth can spread beyond the lung in a process called metastasis into nearby tissue and, eventually, into other parts of the body. Most cancers that start in lung, known as primary...
, ovarian
Ovarian cancer
Ovarian cancer is a cancerous growth arising from the ovary. Symptoms are frequently very subtle early on and may include: bloating, pelvic pain, difficulty eating and frequent urination, and are easily confused with other illnesses....
, breast cancer
Breast cancer
Breast cancer is cancer originating from breast tissue, most commonly from the inner lining of milk ducts or the lobules that supply the ducts with milk. Cancers originating from ducts are known as ductal carcinomas; those originating from lobules are known as lobular carcinomas...
, head and neck cancer, and advanced forms of Kaposi's sarcoma
Kaposi's sarcoma
Kaposi's sarcoma is a tumor caused by Human herpesvirus 8 , also known as Kaposi's sarcoma-associated herpesvirus . It was originally described by Moritz Kaposi , a Hungarian dermatologist practicing at the University of Vienna in 1872. It became more widely known as one of the AIDS defining...
. Paclitaxel is also used for the prevention of restenosis.
Paclitaxel stabilizes microtubules and as a result, interferes with the normal breakdown of microtubules during cell division. Together with docetaxel, it forms the drug category of the taxane
Taxane
The taxanes are diterpenes produced by the plants of the genus Taxus . As their name suggests, they were first derived from natural sources, but some have been synthesized artificially. Taxanes include paclitaxel and docetaxel . Paclitaxel was originally derived from the Pacific yew tree.Taxanes...
s. It was the subject of a notable total synthesis by Robert A. Holton
Robert A. Holton
Robert A. Holton is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol , a widely-utilized and highly-effective anti-cancer drug. He is a Professor of Chemistry at Florida State University. Dr. Holton’s research group has accomplished the total...
.
While offering substantial improvement in patient care, paclitaxel has been a relatively controversial drug. There was originally concern because of the environmental impact of its original sourcing, no longer used, from the Pacific yew. In addition, the assignment of rights, and even the name itself, to Bristol-Myers Squibb were the subject of public debate and Congressional hearings.
Nomenclature
The nomenclature for paclitaxel is structured on a tetracyclic 17-carbon (heptadecaneHeptadecane
Heptadecane is an organic compound, an alkane hydrocarbon with the chemical formula C17H36. The name may refer to any of 24894 theoretically possible constitutional isomers, or to a mixture thereof....
) skeleton. There are a total of 11 stereocenters. The active stereoisomer is (-)-paclitaxel (shown here).
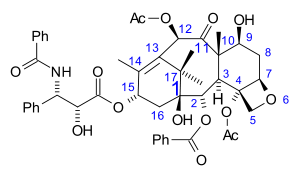 |
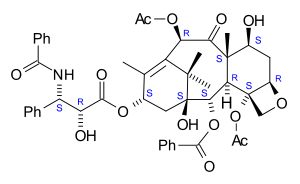 |
(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-Diacetoxy-15-{[(2R,3S)-3- (benzoylamino)-2-hydroxy-3- phenylpropanoyl]oxy}-1,9- dihydroxy-10,14,17,17-tetramethyl -11-oxo-6-oxatetracyclo [11.3.1.0~3,10~.0~4,7~] heptadec-13-en-2-yl rel-benzoate |
The plant screening program, isolation, and preclinical trials
In 1955, the National Cancer InstituteNational Cancer Institute
The National Cancer Institute is part of the National Institutes of Health , which is one of 11 agencies that are part of the U.S. Department of Health and Human Services. The NCI coordinates the U.S...
(NCI) in the United States set up the Cancer Chemotherapy National Service Center (CCNSC) to act as a public screening center for anticancer activity in compounds submitted by external institutions and companies. Although the majority of compounds screened were of synthetic origin, one chemist, Jonathan Hartwell, who was employed there from 1958 onwards, had had experience with natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
derived compounds, and began a plant screening operation. After some years of informal arrangements, in July 1960, the NCI commissioned USDA botanists to collect samples from about 1000 plant species per year. On 21 August 1962, one of those botanists, Arthur S. Barclay, collected bark from a single Pacific yew tree, Taxus brevifolia
Taxus brevifolia
Taxus brevifolia is a conifer native to the Pacific Northwest of North America. It ranges from southernmost Alaska south to central California, mostly in the Pacific Coast Ranges, but with an isolated disjunct population in southeast British Columbia, most notably occurring on Zuckerberg Island...
, in a forest north of the town of Packwood, Washington
Packwood, Washington
Packwood is an unincorporated community located in easternmost Lewis County, Washington.Packwood is located at the intersection of US Highway 12 and Gifford Pinchot National Forest Road 52 . It is located between Mount Rainier National Park to the north and Mt. St. Helens National Volcanic Monument...
as part of a four month trip to collect material from over 200 different species. The material was then processed by a number of specialist CCNSC subcontractors, and one of the Taxus samples was found to be cytotoxic in a cellular assay on 22 May 1964.
Accordingly, in late 1964 or early 1965, the fractionation and isolation laboratory run by Monroe E. Wall in Research Triangle Park
Research Triangle Park
The Research Triangle Park is a research park in the United States. It is located near Durham, Raleigh, and Chapel Hill, in the Research Triangle region of North Carolina...
, North Carolina
North Carolina
North Carolina is a state located in the southeastern United States. The state borders South Carolina and Georgia to the south, Tennessee to the west and Virginia to the north. North Carolina contains 100 counties. Its capital is Raleigh, and its largest city is Charlotte...
, began work on fresh Taxus samples, isolating the active ingredient in September 1966 and announcing their findings at an April 1967 American Chemical Society
American Chemical Society
The American Chemical Society is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 161,000 members at all degree-levels and in all fields of chemistry, chemical...
meeting in Miami Beach. They named the pure compound taxol in June 1967.
Wall and his colleague Wani published their results, including the chemical structure, in 1971.
The NCI continued to commission work to collect more Taxus bark and to isolate increasing quantities of taxol. By 1969, 28 kg of crude extract had been isolated from almost 1,200 kg of bark, although this ultimately yielded only 10g of pure material, but for several years, no use was made of the compound by the NCI. In 1975, it was shown to be active in another in vitro system ; two years later a new department head reviewed the data and finally recommended taxol be moved on to the next stage in the discovery process. This required increasing quantities of purified taxol, up to 600g, and in 1977 a further request for 7,000 lbs of bark was made.
In 1978, two NCI researchers published a report showing taxol was mildly effective in leukaemic mice.
In November 1978, taxol was shown to be effective in xenograft studies.
Meanwhile, taxol began to be well known in the cell biology, as well as the cancer community, with a publication in early 1979 by Susan B. Horwitz, a molecular pharmacologist at Albert Einstein College of Medicine
Albert Einstein College of Medicine
Albert Einstein College of Medicine is a graduate school of Yeshiva University. It is a not-for-profit, private, nonsectarian medical school located on the Jack and Pearl Resnick Campus in the Morris Park neighborhood of the borough of the Bronx of New York City...
, showing taxol had a previously unknown mechanism of action involving the stabilization of microtubules. Together with formulation problems, this increased interest from researchers meant that by 1980, the NCI envisaged needing to collect 20,000 lbs of bark.
Animal toxicology studies were complete by June 1982, and in November NCI applied for the IND
Investigational New Drug
The United States Food and Drug Administration's Investigational New Drug program is the means by which a pharmaceutical company obtains permission to ship an experimental drug across state lines before a marketing application for the drug has been approved...
necessary to begin clinical trials in humans.
Early clinical trials, supply and the transfer to BMS
Phase I clinical trials began in April 1984, and the decision to start Phase IIPhase II
Phase II may refer to:* Phase II clinical trial* Drug metabolism Phase II* Star Trek: Phase II, a planned Star Trek television series that eventually evolved into Star Trek: The Motion Picture...
trials was made a year later. These larger trials needed more bark and collection of a further 12,000 pounds was commissioned, which enabled some phase II trials to begin by the end of 1986. But by then it was recognized that the demand for taxol might be substantial and that more than 60,000 pounds of bark might be needed as a minimum. This unprecedentedly large amount brought ecological concerns about the impact on yew populations into focus for the first time, as local politicians and foresters expressed unease at the program.
The first public report from a phase II trial in May 1988 showed an effect in melanoma patients and a remarkable response rate of 30% in patients with refractory ovarian cancer.
At this point, Gordon Cragg of the NCI's Natural Product Branch calculated the synthesis of enough taxol to treat all the ovarian cancer and melanoma cases in the US would require the destruction of 360,000 trees annually. For the first time, serious consideration was given to the problem of supply.
Because of the practical and, in particular, the financial scale of the program needed, the NCI decided to seek association with a pharmaceutical company, and in August 1989, it published a Cooperative Research and Development Agreement
Cooperative Research and Development Agreement
In the United States, a cooperative research and development agreement is an agreement between a government agency and a private company to work together on research and development.-Description:...
(CRADA) offering its current stock and supply from current bark stocks, and proprietary access to the data so far collected, to a company willing to commit to providing the funds to collect further raw material, isolate taxol, and fund a large proportion of clinical trials. In the words of Goodman and Welsh, authors of a substantial scholarly book on taxol,
[The NCI] was thinking, not of collaboration, ... but of a hand-over of taxol (and its problems)
Although the offer was widely advertised, only four companies responded to the CRADA, including the American firm Bristol-Myers Squibb
Bristol-Myers Squibb
Bristol-Myers Squibb , often referred to as BMS, is a pharmaceutical company, headquartered in New York City. The company was formed in 1989, following the merger of its predecessors Bristol-Myers and the Squibb Corporation...
(BMS),
which was selected as the partner in December 1989. The choice of BMS later became controversial and was the subject of Congressional hearings in 1991 and 1992. While it seems clear the NCI had little choice but to seek a commercial partner, there was also controversy about the terms of the deal, eventually leading to a report by the General Accounting Office in 2003, which concluded the NIH had failed to ensure value for money. In related CRADAs with the USDA and Department of the Interior, Bristol-Myers Squibb was given exclusive first refusal on all Federal supplies of Taxus brevifolia.
This exclusive contract lead to some criticism for giving BMS a "cancer monopoly
Monopoly
A monopoly exists when a specific person or enterprise is the only supplier of a particular commodity...
".
Eighteen months after the CRADA, BMS filed a new drug application
New drug application
The New Drug Application is the vehicle in the United States through which drug sponsors formally propose that the Food and Drug Administration approve a new pharmaceutical for sale and marketing...
(NDA), which was given FDA approval at the very end of 1992.
Although there was no patent on the compound, the provisions of the Waxman-Hatch Act gave Bristol-Myers Squibb five years exclusive marketing rights.
In 1990, BMS applied to trademark the name taxol as TAXOL. This was controversially approved in 1992. At the same time, paclitaxel replaced taxol as the generic name of the compound. Critics, including the journal Nature
Nature
Nature, in the broadest sense, is equivalent to the natural world, physical world, or material world. "Nature" refers to the phenomena of the physical world, and also to life in general...
, argued the name taxol had been used for more than two decades and in more than 600 scientific articles and suggested the trademark should not have been awarded and the BMS should renounce its rights to it. BMS argued changing the name would cause confusion among oncologists and possibly endanger the health of patients. BMS has continued to defend its rights to the name in the courts.
BMS has also been criticized for misrepresentation by Goodman and Walsh, who quote from a company report saying
It was not until 1971 that ... testing ... enabled the isolation of paclitaxel, initially described as 'compound 17'
This quote is, strictly speaking, accurate: the objection seems to be that this misleadingly neglects to explain that it was the scientist doing the isolation who named the compound taxol and it was not referred to in any other way for more than twenty years.
Annual sales peaked in 2000, reaching US$1.6 billion; paclitaxel is now available in generic form.
Production


Pacific Northwest
The Pacific Northwest is a region in northwestern North America, bounded by the Pacific Ocean to the west and, loosely, by the Rocky Mountains on the east. Definitions of the region vary and there is no commonly agreed upon boundary, even among Pacific Northwesterners. A common concept of the...
, that taxol was successfully extracted on a clinically useful scale from these sources.
From the late 1970s, chemists in the US and France had been interested in taxol. A number of US groups, including one led by Robert A. Holton
Robert A. Holton
Robert A. Holton is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol , a widely-utilized and highly-effective anti-cancer drug. He is a Professor of Chemistry at Florida State University. Dr. Holton’s research group has accomplished the total...
, attempted a total synthesis of the molecule, starting from petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
-derived starting materials. This work was primarily motivated as a way of generating chemical knowledge, rather than with any expectation of developing a practical production technique. By contrast, the French group of Pierre Potier at the CNRS quickly recognized the problem of yield. His laboratory was on a campus populated by the related yew Taxus baccata, so needles were available locally in large quantity. By 1981, he had shown that it was feasible to isolate relatively large quantities of the compound 10-deacetylbaccatin
10-deacetylbaccatin
10-Deacetylbaccatins are a series of closely related natural organic compounds isolated from the Pacific yew tree and related species. 10-Deacetylbaccatin III is used as a chemical intermediate in the preparation of the anti-cancer drug paclitaxel ....
, a plausible first step for a semisynthetic production route to taxol. By 1988 he copublished such a semisynthetic route from needles of T. baccata.
The view of the NCI, however, was even this route was not practical.
By 1988, and particularly with Potier's publication, it was clear to Holton as well a practical semisynthetic production route would be important. By late 1989, Holton's group had developed a semisynthetic route to paclitaxel with twice the yield of the Potier process. Florida State University
Florida State University
The Florida State University is a space-grant and sea-grant public university located in Tallahassee, Florida, United States. It is a comprehensive doctoral research university with medical programs and significant research activity as determined by the Carnegie Foundation...
, where Holton worked, signed a deal with Bristol-Myers Squibb
Bristol-Myers Squibb
Bristol-Myers Squibb , often referred to as BMS, is a pharmaceutical company, headquartered in New York City. The company was formed in 1989, following the merger of its predecessors Bristol-Myers and the Squibb Corporation...
to license this and future patents. In 1992, Holton patented an improved process with an 80% yield. BMS took the process in-house and started to manufacture paclitaxel in Ireland from 10-deacetylbaccatin isolated from the needles of the European yew. In early 1993, BMS was able to announce that it would cease reliance on Pacific yew bark by the end of 1995, effectively terminating the ecological controversy over its use. This announcement also made good their commitment to develop an alternative supply route, made to the NCI in their CRADA application of 1989.
Currently, all paclitaxel production for BMS uses plant cell fermentation (PCF) technology developed by the German and Canadian biotechnology company Phyton Biotech, Inc and carried out at their plant in Germany. This starts from a specific Taxus cell line propagated in aqueous medium in large fermentation tanks. Paclitaxel is then extracted directly, purified by chromatography and isolated by crystallization. Compared to the semisynthesis, PCF eliminates the need for many hazardous chemicals and saves a considerable amount of energy.
In 1993, taxol was coincidentally discovered to be produced in a newly described fungus
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
living in the yew tree. It has since been found in a number of other endophytic
Endophyte
An endophyte is an endosymbiont, often a bacterium or fungus, that lives within a plant for at least part of its life without causing apparent disease. Endophytes are ubiquitous and have been found in all the species of plants studied to date; however, most of these endophyte/plant relationships...
fungi, including Nodulisporium sylviforme, Alternaria taxi, Cladosporium cladosporioides MD2, Metarhizium anisopliae, Aspergillus candidus MD3, Mucor rouxianus sp., Chaetomella raphigera, Phyllosticta tabernaemontanae, Phomopsis, Pestalotiopsis pauciseta, Phyllosticta citricarpa, Podocarpus,Fusarium solani, Pestalotiopsis terminaliae, Pestalotiopsis breviseta, Botryodiplodia theobromae Pat., Gliocladium sp., Alternaria alternata var. monosporus, Cladosporium cladosporioides, Nigrospora sp., Pestalotiopsis versicolor, and Taxomyces andreanae, opening the possibility of taxol production by culturing one of these fungal species.
The initial motivation for synthetic approaches to paclitaxel included the opportunity to create closely related compounds. Indeed, this approach led to the development of docetaxel.
Prevalence in Hazelnuts
Recently a group of Italian researchers in the Department of Translational Oncology, National Institute for Cancer Research, IST, Genova with the collaboration of the University of Genova, Italy, has confirmed the presence of taxaneTaxane
The taxanes are diterpenes produced by the plants of the genus Taxus . As their name suggests, they were first derived from natural sources, but some have been synthesized artificially. Taxanes include paclitaxel and docetaxel . Paclitaxel was originally derived from the Pacific yew tree.Taxanes...
s in the shells and leaves of hazel plants, including paclitaxel
Paclitaxel
Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a U.S. National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol...
, 10-deacetylbaccatin III, baccatin III, paclitaxel C, and 7-epipaclitaxel. The finding of these compounds in shells, which are considered discarded material and are mass produced by many food industries, is of interest for the future availability of paclitaxel (Taxol).
Mechanism of action
Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division. Unlike other tubulin-targeting drugs such as colchicineColchicine
Colchicine is a medication used for gout. It is a toxic natural product and secondary metabolite, originally extracted from plants of the genus Colchicum...
that inhibit microtubule assembly, paclitaxel stabilizes the microtubule
Microtubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
polymer and protects it from disassembly. Chromosomes are thus unable to achieve a metaphase spindle configuration. This blocks progression of mitosis, and prolonged activation of the mitotic checkpoint triggers apoptosis or reversion to the G-phase of the cell cycle without cell division,.
The ability of paclitaxel to inhibit spindle function is generally attributed to its suppression of microtubule dynamics, but recent studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. At the higher therapeutic concentrations, paclitaxel appears to suppress microtubule detachment from centrosomes, a process normally activated during mitosis. The binding site for paclitaxel has been identified on the beta-tubulin subunit.
Clinical use
Paclitaxel is approved in the UK for ovarian, breast and lung cancers and Kaposi's sarcomaKaposi's sarcoma
Kaposi's sarcoma is a tumor caused by Human herpesvirus 8 , also known as Kaposi's sarcoma-associated herpesvirus . It was originally described by Moritz Kaposi , a Hungarian dermatologist practicing at the University of Vienna in 1872. It became more widely known as one of the AIDS defining...
.
It is recommended in NICE
NICE
NICE may refer to:* National Independent Cadres and Elites in Iraq* National Institute for Coordinated Experiments, a fictional organisation in C.S...
guidance of June 2001 that it should be used for nonsmall cell lung cancer in patients unsuitable for curative treatment, and in first-line and second-line treatment of ovarian cancer. In September 2001, NICE recommended paclitaxel should be available for the treatment of advanced breast cancer after the failure of anthracyclic chemotherapy, but that its first-line use should be limited to clinical trials. In September 2006, NICE recommended paclitaxel should not be used in the adjuvant treatment of early node-positive breast cancer.
The cost to the NHS per patient in early breast cancer, assuming four cycles of treatment, is about £4000 (approx. $6000).
Similar compounds
AlbuminHuman serum albumin
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin constitutes about half of the blood serum protein...
-bound paclitaxel (trade name Abraxane, also called nab-paclitaxel) is an alternative formulation where paclitaxel is bound to albumin nano-particles.
Much of the clinical toxicity of the original paclitaxel formulation is associated with the solvent Cremophor EL
Cremophor EL
Cremophor EL is the registered trademark of BASF Corp. for its version of polyethoxylated castor oil. It is prepared by reacting 35 moles of ethylene oxide with each mole of castor oil...
in which it is dissolved for delivery.
Much of the clinical toxicity of paclitaxel is associated with the solvent Cremophor EL
Cremophor EL
Cremophor EL is the registered trademark of BASF Corp. for its version of polyethoxylated castor oil. It is prepared by reacting 35 moles of ethylene oxide with each mole of castor oil...
in which it is dissolved for delivery.
Abraxis BioScience
Abraxis BioScience
Abraxis BioScience is a global biopharmaceutical company dedicated to meeting the needs of critically ill patients, with over 2000 employees worldwide.-Description:...
developed Abraxane, in which paclitaxel is bonded to albumin
Human serum albumin
Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver. Albumin constitutes about half of the blood serum protein...
as an alternative delivery agent to the often toxic solvent delivery method. This was approved by the U.S. Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
in January 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within six months of adjuvant chemotherapy.
The closely related taxane docetaxel has a similar set of clinical uses to paclitaxel. It is marketed under the name of Taxotere.
Restenosis
Paclitaxel is used as an antiproliferative agent for the prevention of restenosisRestenosis
Restenosis literally means the reoccurrence of stenosis, a narrowing of a blood vessel, leading to restricted blood flow. Restenosis usually pertains to an artery or other large blood vessel that has become narrowed, received treatment to clear the blockage and subsequently become renarrowed...
(recurrent narrowing) of coronary stent
Stent
In the technical vocabulary of medicine, a stent is an artificial 'tube' inserted into a natural passage/conduit in the body to prevent, or counteract, a disease-induced, localized flow constriction. The term may also refer to a tube used to temporarily hold such a natural conduit open to allow...
s; locally delivered to the wall of the coronary artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents. Paclitaxel drug eluting coated stents
Drug-eluting stent
A drug-eluting stent ' is a peripheral or coronary stent placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots , could otherwise block the stented artery, a process called restenosis...
are sold under the trade name Taxus by Boston Scientific
Boston Scientific
The Boston Scientific Corporation , is a worldwide developer, manufacturer and marketer of medical devices whose products are used in a range of interventional medical specialties, including interventional cardiology, peripheral interventions, neuromodulation, neurovascular intervention,...
in the United States.
Side effects
Common side effects include nausea and vomiting, loss of appetite, change in taste, thinned or brittle hair, pain in the joints of the arms or legs lasting two to three days, changes in the color of the nails, and tingling in the hands or toes. More serious side effects such as unusual bruising or bleeding, pain/redness/swelling at the injection site, change in normal bowel habits for more than two days, fever, chills, cough, sore throat, difficulty swallowing, dizziness, shortness of breath, severe exhaustion, skin rash, facial flushing, female infertilityFemale infertility
-Causes and factors:Causes or factors of female infertility can basically be classified regarding whether they are acquired or genetic, or strictly by location.-Acquired versus genetic:...
by ovarian damage and chest pain can also occur. A number of these side effects are associated with the excipient
Excipient
An excipient is generally a pharmacologically inactive substance used as a carrier for the active ingredients of a medication. In many cases, an "active" substance may not be easily administered and absorbed by the human body; in such cases the substance in question may be dissolved into or...
used, Cremophor EL, a polyoxyethylated castor oil
Castor oil
Castor oil is a vegetable oil obtained from the castor bean . Castor oil is a colorless to very pale yellow liquid with mild or no odor or taste. Its boiling point is and its density is 961 kg/m3...
. Allergies to drugs such as cyclosporine, teniposide and drugs containing polyoxyethylated castor oil may indicate increased risk of adverse reactions to paclitaxel. Dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
is given prior to beginning paclitaxel treatment to mitigate some of the side effects. Leuprolide
Leuprolide
Leuprorelin or leuprolide acetate is a GnRH analog. Proper Sequence: Pyr-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt - Mode of action:Leuprolide acts as an agonist at pituitary GnRH receptors...
, a GnRH analog
Gonadotropin-releasing hormone analogue
A gonadotropin-releasing hormone analogue , also known as a luteinizing hormone releasing hormone agonist or LHRH analogue is a synthetic peptide drug modeled after the human hypothalamic gonadotropin-releasing hormone...
may prevent ovarian damage, according to mice studies.
Derivatives of paclitaxel
In recent years, extensive research has been done to find a way to mitigate the side effects of paclitaxel, by altering its administration. DHA-paclitaxel, PG-paclitaxel, and tumor-activated Taxol prodrugs are undergoing continued testing, and are actually on the way to being introduced into widespread clinical use.Protarga has linked paclitaxel to docosahexaenoic acid
Docosahexaenoic acid
Docosahexaenoic acid is an omega-3 fatty acid that is a primary structural component of the human brain and retina. In chemical structure, DHA is a carboxylic acid with a 22-carbon chain and six cis double bonds; the first double bond is located at the third carbon from the omega end...
(DHA), a fatty acid easily taken up by tumor cells; the DHA-paclitaxel
DHA-paclitaxel
DHA-paclitaxel is an investigational drug made by linking paclitaxel to docosahexaenoic acid , a fatty acid that is easily taken up by tumor cells; the DHA-paclitaxel “appears not to be cytotoxic until the bond with DHA is cleaved within the cell.” The advantage of DHA-paclitaxel over paclitaxel...
“appears not to be cytotoxic until the bond with DHA is cleaved within the cell.” The advantage of DHA-paclitaxel over paclitaxel is DHA-paclitaxel’s ability to carry much higher concentrations of paclitaxel to the cells, which are maintained for longer periods in the tumor cells, thus increasing their action. With increased activity, DHA-paclitaxel, also known as Taxoprexin, may have a more successful response in cancer patients than paclitaxel, and it may be able to treat more types of cancer than paclitaxel has been able to treat.
Cell Therapeutics has formulated PG-paclitaxel, which is paclitaxel bonded to a polyglutamate polymer; tumor cells are significantly more porous to polyglutamate polymers than normal cells, due to the leaky endothelial membranes of tumor cells. PG-paclitaxel has been introduced into clinical use, and has proven to initiate very mild side effects and to effectively treat many patients who were not responsive to the action of Taxol. The PG-paclitaxel may be a very promising anticancer drug, as it is much more selective than paclitaxel for which cells it targets.
ImmunoGen has been introducing tumor-activated prodrug (TAP) technology in recent years, and is now working to apply this technology to paclitaxel. Tumor-activated Taxol prodrugs are designed for accurate targeting, by the action of a monoclonal antibody which is very specific to certain cells. Tumor-activated Taxol prodrugs research is progressing, and in mice, the “taxane-based TAP completely eradicated human tumour xenografts at non-toxic doses.”
ANG1005
ANG1005
ANG1005 is a drug candidate engineered to cross the blood brain barrier to deliver chemotherapy for the treatment of glioma.It is made up of one molecule of a peptide called Angiopep-2 joined together with three molecules of Paclitaxel, a taxane-chemotherapy drug...
is made up of one molecule of a peptide called angiopep-2 joined with three molecules of paclitaxel. It is in phase I clinical trials for some types of cancer.
Research use
Aside from its direct clinical use, paclitaxel is used extensively in biological and biomedical research as a microtubuleMicrotubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
stabilizer. In vitro
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
assays involving microtubules, such as motility assays, generally rely on paclitaxel to maintain microtubule integrity in the absence of the various nucleating factors and other stabilizing elements found in the cell. For example, it is used for in vitro tests of drugs that aim to alter the behavior of microtubule motor proteins, or for studies of mutant motor proteins. Paclitaxel is sometimes used for in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
studies as well; it can be fed to test organisms, such as fruit flies
Drosophila melanogaster
Drosophila melanogaster is a species of Diptera, or the order of flies, in the family Drosophilidae. The species is known generally as the common fruit fly or vinegar fly. Starting from Charles W...
, or injected into individual cells, to inhibit microtubule disassembly or to increase the number of microtubules in the cell. Paclitaxel induces remyelination in a demyelinating mouse in vivo and inhibits hPAD2 in vitro though its methyl ester side chain did not. Angiotech Pharmaceuticals Inc. began phase II clinical trials in 1999 as a multiple sclerosis treatment but in 2002, reported that the results showed no statistical significance.
Biosynthesis and Biocatalysis
The core synthetic route is via an terpenoidTerpenoid
The terpenoids , sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in...
pathway, parts of which have been successfully transplanted into production strains of E.coli and yeast
External links
- NCI Drug Information Summary for Patients.
- NCI Drug Dictionary Definition
- Molecule of the Month: TAXOL by Neil Edwards, University of BristolUniversity of BristolThe University of Bristol is a public research university located in Bristol, United Kingdom. One of the so-called "red brick" universities, it received its Royal Charter in 1909, although its predecessor institution, University College, Bristol, had been in existence since 1876.The University is...
. - A Tale of Taxol from Florida State UniversityFlorida State UniversityThe Florida State University is a space-grant and sea-grant public university located in Tallahassee, Florida, United States. It is a comprehensive doctoral research university with medical programs and significant research activity as determined by the Carnegie Foundation...
. - Anzatax / Paclitaxel Virtual Cancer Centre

