
Parikh-Doering oxidation
Encyclopedia
The Parikh-Doering
oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses DMSO
as the oxidant, activated by sulfur trioxide-pyridine complex in the presence of triethylamine base:
 The procedure can be run at temperatures close to ambient (usually 0°C) without formation of significant amounts of methylthiomethyl ether side product.
The procedure can be run at temperatures close to ambient (usually 0°C) without formation of significant amounts of methylthiomethyl ether side product.
The following example from the total synthesis of (–)-kumausallene by P.A. Evans and coworkers illustrates typical reaction conditions:
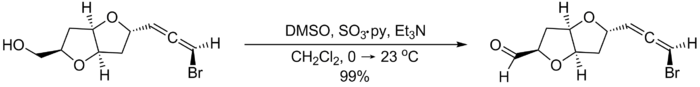
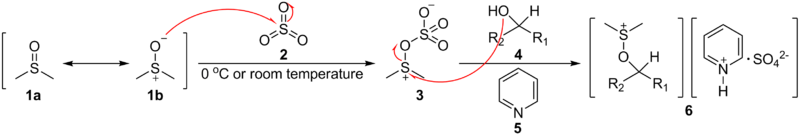
(DMSO), 1a & 1b, with sulfur trioxide
, 2 at 0 oC or room temperature. Then, the intermediate 3 receives a nucleophilic addition from the alcohol
to give the key alkoxysulfonium ion intermediate, 6, where the counterweight of positive charge is sulfate
coordinated by pyridine
.
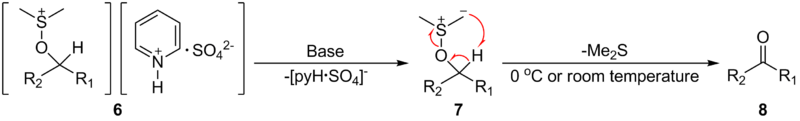
 After that, the addition of at least 2 equivalents of base — typically triethylamine
After that, the addition of at least 2 equivalents of base — typically triethylamine
— will deprotonate the alkoxysulfonium ion to give the sulfur ylide
7. The base also helps the charge counterweight to leave. In the last step, through a five-membered ring transition state
, the sulfur ylide 7 decomposes to give the desired ketone or aldehyde 8.
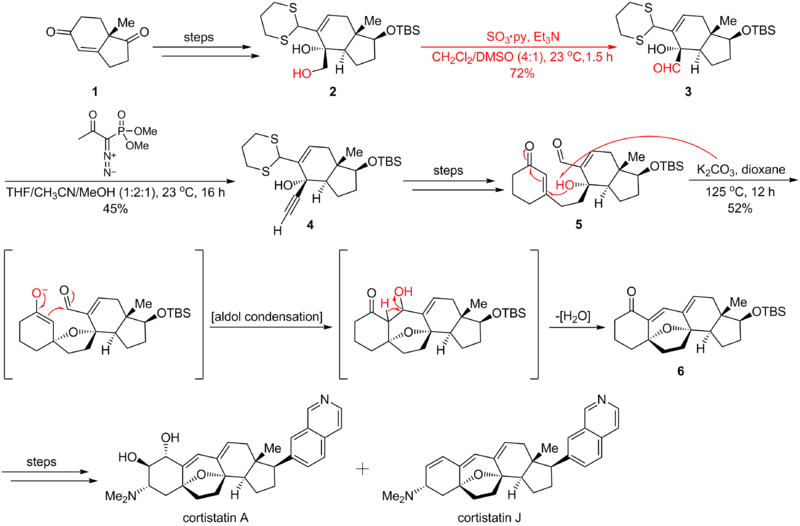
which is critical in following 1,4 addition/aldol condensation/dehydration cascade constructing the cortistatins'
seven-membered ring. The synthetic route is shown as below:
William von Eggers Doering
William von Eggers Doering was a Professor Emeritus at Harvard University and the former Chair of its Chemistry Department...
oxidation is an oxidation reaction that transforms primary and secondary alcohols into aldehydes and ketones, respectively. The procedure uses DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
as the oxidant, activated by sulfur trioxide-pyridine complex in the presence of triethylamine base:

The following example from the total synthesis of (–)-kumausallene by P.A. Evans and coworkers illustrates typical reaction conditions:
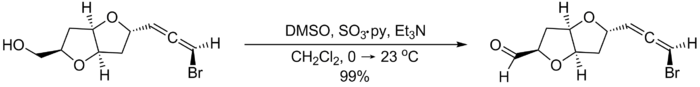
Mechanism
The first step of the Parikh-Doering oxidation is the reaction of dimethyl sulfoxideDimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
(DMSO), 1a & 1b, with sulfur trioxide
Sulfur trioxide
Sulfur trioxide is the chemical compound with the formula SO3. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain. It is prepared on massive scales as a precursor to sulfuric acid.-Structure and bonding:Gaseous SO3 is a trigonal planar molecule of...
, 2 at 0 oC or room temperature. Then, the intermediate 3 receives a nucleophilic addition from the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
to give the key alkoxysulfonium ion intermediate, 6, where the counterweight of positive charge is sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
coordinated by pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
.

Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
— will deprotonate the alkoxysulfonium ion to give the sulfur ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
7. The base also helps the charge counterweight to leave. In the last step, through a five-membered ring transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
, the sulfur ylide 7 decomposes to give the desired ketone or aldehyde 8.

Strategic Application
Parikh-Doering oxidation is widely applied in organic synthesis. Here is an example of Parikh-Doering oxidation's application in Nicolaou cortistatin total synthesis where the reaction transforms the hydroxyl function group into a aldehyde group. This process leads to Ohira-Bestmann homologationSeyferth-Gilbert homologation
The Seyferth–Gilbert homologation is a the chemical reaction of an aryl ketone 1 with dimethyl phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3...
which is critical in following 1,4 addition/aldol condensation/dehydration cascade constructing the cortistatins'
Cortistatins
The cortistatins are a group of steroidal alkaloids first isolated in 2006 from the marine sponge Corticium simplex. The cortistatins inhibit proliferation of human umbilical vein endothelial cells with high selectivity, with cortistatin A being the most potent compound in the class.The unique...
seven-membered ring. The synthetic route is shown as below:


