
Partial oxidation
Encyclopedia
Partial oxidation is a type of chemical reaction
. It occurs when a substoichiometric
fuel-air mixture is partially combusted
in a reformer, creating a hydrogen-rich syngas
which can then be put to further use, for example in a fuel cell
. A distinction is made between thermal partial oxidation (TPOX) and catalytic partial oxidation (CPOX).
or a heavy hydrocarbon (heating oil
) is mixed in an exothermic
process with oxygen.
The formulas given for coal and heating oil show only a typical representative of these highly complex mixtures. Water is added to the process for getting both the extreme temperatures as well as extra control on the formation of soot.
, proceed at temperatures of 1200°C
and above.
The choice of reforming
technique depends on the sulfur
content of the fuel being used. CPOX can be employed if the sulfur content is below 50 ppm. A higher sulfur content can poison the catalyst, so the TPOX procedure is used for such fuels. However, recent research shows that CPOX is posible with sulfur contents up to 400ppm .
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. It occurs when a substoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
fuel-air mixture is partially combusted
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
in a reformer, creating a hydrogen-rich syngas
Syngas
Syngas is the name given to a gas mixture that contains varying amounts of carbon monoxide and hydrogen. Examples of production methods include steam reforming of natural gas or liquid hydrocarbons to produce hydrogen, the gasification of coal, biomass, and in some types of waste-to-energy...
which can then be put to further use, for example in a fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
. A distinction is made between thermal partial oxidation (TPOX) and catalytic partial oxidation (CPOX).
Principle
Partial oxidation is a technically mature process in which natural gasNatural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
or a heavy hydrocarbon (heating oil
Heating oil
Heating oil, or oil heat, is a low viscosity, flammable liquid petroleum product used as a fuel for furnaces or boilers in buildings. Home heating oil is often abbreviated as HHO...
) is mixed in an exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
process with oxygen.
- General reaction equation (without catalyst, TPOX):

- General reaction equation (with catalyst, CPOX):
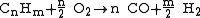
- Possible reaction equation (heating oil):
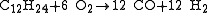
- Possible reaction equation (coal):

The formulas given for coal and heating oil show only a typical representative of these highly complex mixtures. Water is added to the process for getting both the extreme temperatures as well as extra control on the formation of soot.
TPOX
TPOX (thermal partial oxidation) reactions, which are dependent on the air-fuel ratioAir-fuel ratio
Air–fuel ratio is the mass ratio of air to fuel present in an internal combustion engine. If exactly enough air is provided to completely burn all of the fuel, the ratio is known as the stoichiometric mixture, often abbreviated to stoich...
, proceed at temperatures of 1200°C
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
and above.
CPOX
In CPOX (catalytic partial oxidation) the use of a catalyst reduces the required temperature to around 800°C – 900°C.The choice of reforming
Catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas, typically having low octane ratings, into high-octane liquid products called reformates which are components of high-octane gasoline...
technique depends on the sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
content of the fuel being used. CPOX can be employed if the sulfur content is below 50 ppm. A higher sulfur content can poison the catalyst, so the TPOX procedure is used for such fuels. However, recent research shows that CPOX is posible with sulfur contents up to 400ppm .
History
1926 – Vandeveer and Parr at the University of Illinois used oxygen in the place of air.See also
- Hydrogen productionHydrogen productionHydrogen production is the family of industrial methods for generating hydrogen. Currently the dominant technology for direct production is steam reforming from hydrocarbons. Many other methods are known including electrolysis and thermolysis...
- IPOX (indirect partial oxidation)
- PROXPROXPROX is an acronym for PReferential OXidation, and refers to the preferential oxidation of a gas on a catalyst.The catalyser preferentially oxidises carbon monoxide using a heterogeneous catalyst placed upon a ceramic support...
- Glossary of fuel cell termsGlossary of fuel cell termsThe Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. –...
- Timeline of hydrogen technologiesTimeline of hydrogen technologiesTimeline of hydrogen technologies — A timeline of the history of hydrogen technology.-1600s:* 1625 - First description of hydrogen by Johann Baptista van Helmont...

