
Parylene
Encyclopedia
Parylene is the tradename for a variety of chemical vapor deposited poly(p-xylylene
) polymers used as moisture and dielectric barriers. Among them, Parylene C is the most popular due to its combination of barrier properties, cost, and other processing advantages.
Parylene is green polymer chemistry
. It is self-initiated (no initiator needed) and un-terminated (no termination group needed) with no solvent or catalyst required. The commonly used precursor, [2.2]paracyclophane, yields 100% monomer above 550 °C in vacuum and the initiator and does not yield any by-products. That said there are alternative precursors to arrive at the parylene polymers that possess leaving groups as opposed to the cyclophane precursor. The most popular using bromine to yield the parylene AF-4 polymer. However, bromine is corrosive towards most metals and metal alloys and Viton O-rings so it is difficult to work with and precautions are needed.
Parylene C and to a lesser extent AF-4, SF, HT (all the same polymer) are used for coating printed circuit board
s (PCBs) and medical device
s. There are numerous other applications as parylene is an excellent moisture barrier. It is the most bio-accepted coating for stents, defibrillators, pacemakers and other devices permanently implanted into the body.
Parylenes are relatively soft (0.25 GPa) except for Parylene X (1.0 GPa) and they have poor oxidative resistance (~115 °C) and UV stability, except for Parylene AF-4. However, Parylene AF-4 is more expensive due to a three-step synthesis of its precursor with low yield and a poor deposition efficiency. Their UV stability is so poor that parylene cannot be exposed to regular sunlight without yellowing.
Nearly all the parylenes are insoluble at room temperature except for the alkylated parylenes, one of which is parylene E. This lack of solubility has made it difficult to re-work printed circuit boards coated with parylene.
Copolymers and Nanocomposites (SiO2/parylene C) of parylene have been deposited at near-room temperature previously; and with strongly electron withdrawing comonomers, parylene can be used as an initiator to initiate polymerizations, such as with N-phenyl maleimide
. Using the parylene C/SiO2 nanocomposites, parylene C could be used as a sacrificial layer to make nanoporous silica thin films with a porosity of >90%.
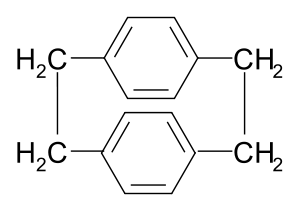 Parylene N is a polymer manufactured (chemical vapor deposited) from the p-xylylene intermediate. The p-xylylene intermediate is commonly derived from [2.2]paracyclophane
Parylene N is a polymer manufactured (chemical vapor deposited) from the p-xylylene intermediate. The p-xylylene intermediate is commonly derived from [2.2]paracyclophane
. The latter compound can be synthesized from p-xylene involving several steps involving bromination, amination
and Hofmann elmination.
Parylene N is an unsubstituted molecule. Heating [2.2]paracyclophane under low pressure (0.001 – 0.1 Torr) conditions gives rise to the p-xylylene intermediate
which polymerizes
when physisorbed
on a surface. The p-xylylene intermediate has two quantum mechanical states, the benzoid state (triplet state) and the quinoid state (singlet state). The triplet state is effectively the initiator and the singlet state is effectively the monomer. The triplet state can be de-activated when in contact with transition metals or metal oxides including Cu/CuOx. Many of the parylenes exhibit this selectivity based on quantum mechanical deactivation of the triplet state, including parylene X. However, like any selective process there is a 'selectivity' window based on mostly deposition pressure and deposition temperature for the paryelne polymers. What is more, the intermediate, p-xylylene has a low reactivity and therefore a small 'sticking coefficient' and as a result parylene N produces a highly conformal thin film or coating.
The deposition of parylene N is a function of a two-step process. First, physisorption needs to take place, which is a function of deposition pressure and temperature. The physisorption has inverse Arrhenius kinetics
, in other words it is stronger at lower temperatures than higher temperatures. All the parylenes have a critical temperature called the threshold temperature above which practically no deposition is observed. The closer the deposition temperature is to the threshold temperature the weaker the physisorption. Once physisorption occurs than the p-xylylene intermediate needs to react with itself (2nd step) for polymerization to occur. For parylene N, its threshold temperature is 40 °C.
There are a couple of fluorinated parylenes commercially available, parylene AF-4 (generic name, aliphatic flourination 4 atoms) [parylene SF (AF-4, Kisco product), parylene HT (AF-4, SCS product)] and parylene VT-4 (generic name, fluorine atoms on the aromatic ring) [also Parylene CF (VT-4, Kisco product)]. Parylene AF-4 is very expensive due to its inefficient wet chemical synthesis of its precursor and its inefficient deposition due to its low polarizability. Polarizability ultimately determines how strongly the intermediate chemistry interacts with the surface and polarizability strongly correlates with molecular weight of the intermediate except in the case of the fluorinated chemistries. Parylene AF-4 is a PTFE analogue in the sense that its aliphatic chemistry has the repeat unit -CF2- and as a result has superior oxidative and UV stability. In contrast, parylene VT-4 (sometimes called just parylene F) has the aliphatic -CH2- chemistry and therefore has poor oxidative and UV stability. Parylene AF-4 has been used to protect outdoor LED
displays and lighting from water, salt and pollutants successfully.
The standard Gorham process regardless of the cyclophane starting chemistry is shown above for parylene AF-4. The octafluoro[2.2]paracyclophane is generally sublimed below <100 °C via different configurations. The cyclophane is transported to a pyrolysis zone where it is 'cracked' to the p-xylylene intermediate. This temperature is generally 700 °C, higher than the temperature (650 °C) used to crack the hydrocarbon cyclophane since the -CF2-CF2- bond is stronger than the -CH2-CH2- bond. This resonance-stabilized intermediate is transported to a room temperature deposition chamber where polymerization is able to occur under low pressure (1–100 mTorr) conditions. The threshold temperature of parylene AF-4 is very close to room temperature (30–35 °C), as a result, its deposition efficiency is poor.
More recently an alternate route to parylene AF-4 was developed as shown above. The advantage to this process is the low cost of synthesis for the precursor. The precursor is also a liquid and can be delivered by standard methods developed in the Semiconductor Industry, such as with a vaporizer or vaporizer with a bubbler. Originally the precursor was just thermally cracked to yield the same intermediate as that produced from the cyclophane; however, with the use of catalysts the 'cracking' temperature can be lowered resulting in less char in the pyrolysis zone and a higher quality polymer thin film. By either method free radical bromine is given off as a by-product and is easily converted to hydrogen bromide, which has to be properly processed or equipment damage will occur.
Among all the parylenes, parylene X is especially unique since it is: 1) cross-link
able (thermally or with UV-light) 2) Can generate the Cu-acetylide
or Ag-acetylide
metallorganic intermediates 3) Can undergo 'Click chemistry
' 4) Can be used as an adhesive
, parylene-to-parylene bonding without any by-products during processing 5) Is amorphous
(non-crystalline) and is 6) A hydrocarbon polymer.
Polymeric surfaces generally only possess dispersion forces but may contain functional groups able to bond to adhesion promoters. If parylene is bonded to a printed circuit board (PCB) then often the mechanical tie-points allow parylene to exhibit good adhesion as opposed to bonding through covalent links (chemical bonding). Sometimes plasma methods are effective in the promotion of adhesion between parylene and polymeric surfaces but these techniques are not trivial to employ. The third surface, metal-oxide forming surfaces, generally possess a hydroxyl-terminated surface, M-OH, where M is a metal such as aluminum or chromium. This termination group has the ability to react with commercially available silanes such as A-174 (methacryloxypropyltrimethoxysilane), which is the common adhesion promoter for the parylene polymers.
The A-174 silane can be vapor delivered in situ or bonded via wet chemical baths. In all cases one half of the molecule binds to metal oxide forming surface through sol-gel chemistry (hydrolysis and condensation) and the other half co-polymerizes with parylene via a free radical addition
reaction. In all cases the A-174 silane molecule 'lies down' on the surface and forms self-limited molecular layers of less than 1.0 nm. If thick layers are observed than the silane bath has started to 'polymerize' and a new bath should be started. Vapor phase silylation never yields more than a sub-monolayer of silane on the part being coated; and therefore this problem is circumvented.
discovered the polymer as one of the thermal decomposition products of a common solvent p-xylene
at a temperatures exceeding 1000 °C. Szwarc first postulated the monomer to be para-xylylene which he confirmed by reacting the vapors with iodine and observing the para-xylylene di-iodide as the only product. The reaction yield was only a few percent, and a more efficient route was found later by William F. Gorham at Union Carbide. He deposited parylene films by the thermal decomposition of [2.2] paracyclophane at temperatures exceeding 550 °C and in vacuum below 1 Torr. This process did not require a solvent and resulted in chemically resistant films free from pinholes. Since the coating process takes place at ambient temperature in a mild vacuum, and because of parylene’s conformal properties, it has a wide variety of applications. Union Carbide commercialized a parylene coating system in 1965. Union Carbide went onto undertake research into the synthesis of numerous parylene precursors, including parylene AF-4, throughout the 1960s into the early 70's. There legacy lived on with NovaTran (now defunct) and now with Specialty Coating Systems. Very little innovation with the parylene polymers has been undertaken since via any of the commercial parylene companies. However, much university research utilizes the unique parylene polymers and their unique deposition process.
Xylylene
Xylylene comprises two isomeric organic compounds with the formula C6H42. These compounds are related to the corresponding quinones by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form...
) polymers used as moisture and dielectric barriers. Among them, Parylene C is the most popular due to its combination of barrier properties, cost, and other processing advantages.
Parylene is green polymer chemistry
Green chemistry
Green chemistry, also called sustainable chemistry, is a philosophy of chemical research and engineering that encourages the design of products and processes that minimize the use and generation of hazardous substances...
. It is self-initiated (no initiator needed) and un-terminated (no termination group needed) with no solvent or catalyst required. The commonly used precursor, [2.2]paracyclophane, yields 100% monomer above 550 °C in vacuum and the initiator and does not yield any by-products. That said there are alternative precursors to arrive at the parylene polymers that possess leaving groups as opposed to the cyclophane precursor. The most popular using bromine to yield the parylene AF-4 polymer. However, bromine is corrosive towards most metals and metal alloys and Viton O-rings so it is difficult to work with and precautions are needed.
Parylene C and to a lesser extent AF-4, SF, HT (all the same polymer) are used for coating printed circuit board
Printed circuit board
A printed circuit board, or PCB, is used to mechanically support and electrically connect electronic components using conductive pathways, tracks or signal traces etched from copper sheets laminated onto a non-conductive substrate. It is also referred to as printed wiring board or etched wiring...
s (PCBs) and medical device
Medical device
A medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
s. There are numerous other applications as parylene is an excellent moisture barrier. It is the most bio-accepted coating for stents, defibrillators, pacemakers and other devices permanently implanted into the body.
Parylenes are relatively soft (0.25 GPa) except for Parylene X (1.0 GPa) and they have poor oxidative resistance (~115 °C) and UV stability, except for Parylene AF-4. However, Parylene AF-4 is more expensive due to a three-step synthesis of its precursor with low yield and a poor deposition efficiency. Their UV stability is so poor that parylene cannot be exposed to regular sunlight without yellowing.
Nearly all the parylenes are insoluble at room temperature except for the alkylated parylenes, one of which is parylene E. This lack of solubility has made it difficult to re-work printed circuit boards coated with parylene.
Copolymers and Nanocomposites (SiO2/parylene C) of parylene have been deposited at near-room temperature previously; and with strongly electron withdrawing comonomers, parylene can be used as an initiator to initiate polymerizations, such as with N-phenyl maleimide
Maleimide
Maleimide is the chemical compound with the formula H2C22NH . This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -CNHC- functional group...
. Using the parylene C/SiO2 nanocomposites, parylene C could be used as a sacrificial layer to make nanoporous silica thin films with a porosity of >90%.
Parylene N

Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
. The latter compound can be synthesized from p-xylene involving several steps involving bromination, amination
Amination
Amination is the process by which an amine group is introduced into an organic molecule. Enzymes which catalyse this reaction, are termed aminases. This can occur in a number of ways including reaction with ammonia or another amine such as an alkylation, reductive amination and the Mannich reaction...
and Hofmann elmination.
Parylene N is an unsubstituted molecule. Heating [2.2]paracyclophane under low pressure (0.001 – 0.1 Torr) conditions gives rise to the p-xylylene intermediate
which polymerizes
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
when physisorbed
Physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption...
on a surface. The p-xylylene intermediate has two quantum mechanical states, the benzoid state (triplet state) and the quinoid state (singlet state). The triplet state is effectively the initiator and the singlet state is effectively the monomer. The triplet state can be de-activated when in contact with transition metals or metal oxides including Cu/CuOx. Many of the parylenes exhibit this selectivity based on quantum mechanical deactivation of the triplet state, including parylene X. However, like any selective process there is a 'selectivity' window based on mostly deposition pressure and deposition temperature for the paryelne polymers. What is more, the intermediate, p-xylylene has a low reactivity and therefore a small 'sticking coefficient' and as a result parylene N produces a highly conformal thin film or coating.
The deposition of parylene N is a function of a two-step process. First, physisorption needs to take place, which is a function of deposition pressure and temperature. The physisorption has inverse Arrhenius kinetics
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
, in other words it is stronger at lower temperatures than higher temperatures. All the parylenes have a critical temperature called the threshold temperature above which practically no deposition is observed. The closer the deposition temperature is to the threshold temperature the weaker the physisorption. Once physisorption occurs than the p-xylylene intermediate needs to react with itself (2nd step) for polymerization to occur. For parylene N, its threshold temperature is 40 °C.
Common halogenated parylenes
Parylene N can be derivatived with respect to its main-chain phenyl ring and its aliphatic carbon bonds. The most common parylene is parylene C (one chlorine group per repeat unit, as shown above) followed by parylene D (two chlorine groups per repeat unit); both chlorine groups are on the main-chain phenyl ring. Due to its higher molecular weight parylene C has a higher threshold temperature, 90 °C, and therefore has a much higher deposition rate, while still possessing a high degree of conformality. It can be deposited at room temperature while still possessing a high degree of conformality and uniformity and a moderate deposition rate >1 nm/s in a batch process. As a moisture diffusion barrier, the efficacy of coatings scale non-linearly with their density. Halogen atoms such as F, Cl, and Br add much density to the coating and therefore allow the coating to be a better diffusion barrier. In that regard parylene D is a better diffusion barrier compared to parylene C; however, parylene D suffers from poor across-the-chamber uniformity and conformality at room temperature due to its high molecular weight (135 °C threshold temperature), as a result it is used much less than parylene C.There are a couple of fluorinated parylenes commercially available, parylene AF-4 (generic name, aliphatic flourination 4 atoms) [parylene SF (AF-4, Kisco product), parylene HT (AF-4, SCS product)] and parylene VT-4 (generic name, fluorine atoms on the aromatic ring) [also Parylene CF (VT-4, Kisco product)]. Parylene AF-4 is very expensive due to its inefficient wet chemical synthesis of its precursor and its inefficient deposition due to its low polarizability. Polarizability ultimately determines how strongly the intermediate chemistry interacts with the surface and polarizability strongly correlates with molecular weight of the intermediate except in the case of the fluorinated chemistries. Parylene AF-4 is a PTFE analogue in the sense that its aliphatic chemistry has the repeat unit -CF2- and as a result has superior oxidative and UV stability. In contrast, parylene VT-4 (sometimes called just parylene F) has the aliphatic -CH2- chemistry and therefore has poor oxidative and UV stability. Parylene AF-4 has been used to protect outdoor LED
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
displays and lighting from water, salt and pollutants successfully.
The standard Gorham process regardless of the cyclophane starting chemistry is shown above for parylene AF-4. The octafluoro[2.2]paracyclophane is generally sublimed below <100 °C via different configurations. The cyclophane is transported to a pyrolysis zone where it is 'cracked' to the p-xylylene intermediate. This temperature is generally 700 °C, higher than the temperature (650 °C) used to crack the hydrocarbon cyclophane since the -CF2-CF2- bond is stronger than the -CH2-CH2- bond. This resonance-stabilized intermediate is transported to a room temperature deposition chamber where polymerization is able to occur under low pressure (1–100 mTorr) conditions. The threshold temperature of parylene AF-4 is very close to room temperature (30–35 °C), as a result, its deposition efficiency is poor.
More recently an alternate route to parylene AF-4 was developed as shown above. The advantage to this process is the low cost of synthesis for the precursor. The precursor is also a liquid and can be delivered by standard methods developed in the Semiconductor Industry, such as with a vaporizer or vaporizer with a bubbler. Originally the precursor was just thermally cracked to yield the same intermediate as that produced from the cyclophane; however, with the use of catalysts the 'cracking' temperature can be lowered resulting in less char in the pyrolysis zone and a higher quality polymer thin film. By either method free radical bromine is given off as a by-product and is easily converted to hydrogen bromide, which has to be properly processed or equipment damage will occur.
Reactive parylenes
Most parylenes are passivation thin films or coatings. This means they protect the device or part from environmental stresses such as water, chemical attack, or applied field. This is an important property however many applications have the need to bond other materials to parylene, bond parylene to parylene, or even immobilize catalysts or enzymes to the parylene surface. Some of the reactive parylenes are parylene A (one amine per repeat unit, Kisco product), parylene AM (one methylene amine group per repeat unit, Kisco product), and parylene X (a reactive hydrocarbon cross-linkable version, not commercially available). Parylene AM is more reactive than A since it is a stronger base. When adjacent to the phenyl ring the amine group, -NH2-, is in resonance stabilization and therefore become more acidic and a result less reactive as a base. However, parylene A is much easier to synthesize and hence it costs less.Among all the parylenes, parylene X is especially unique since it is: 1) cross-link
Cross-link
Cross-links are bonds that link one polymer chain to another. They can be covalent bonds or ionic bonds. "Polymer chains" can refer to synthetic polymers or natural polymers . When the term "cross-linking" is used in the synthetic polymer science field, it usually refers to the use of...
able (thermally or with UV-light) 2) Can generate the Cu-acetylide
Copper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
or Ag-acetylide
Silver acetylide
Silver acetylide is an inorganic chemical compound with the formula Ag2C2, a metal acetylide. The name is derived from the way it is synthesized, and emphasizes that the compound can be regarded as a salt of the weak acid, acetylene. Since acetylene is called "ethyne" in the modern IUPAC...
metallorganic intermediates 3) Can undergo 'Click chemistry
Click chemistry
Click chemistry is a chemical philosophy introduced by K. Barry Sharpless of The Scripps Research Institute, in 2001 and describes chemistry tailored to generate substances quickly and reliably by joining small units together...
' 4) Can be used as an adhesive
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
, parylene-to-parylene bonding without any by-products during processing 5) Is amorphous
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
(non-crystalline) and is 6) A hydrocarbon polymer.
Adhesion
The majority of parylene used is deposited as passivation coatings to passivate the part or device towards moisture, chemical attack or as a dielectric insulator. This in turn often means parylene is coated over complex topographies with many different surface chemistries. If one considers a solid-state material, those materials have three fundamental surfaces when exposed to ambient conditions: 1) noble metal surfaces, 2) metal-oxide forming surfaces, and 3) organic surfaces, e.g. polymeric.Polymeric surfaces generally only possess dispersion forces but may contain functional groups able to bond to adhesion promoters. If parylene is bonded to a printed circuit board (PCB) then often the mechanical tie-points allow parylene to exhibit good adhesion as opposed to bonding through covalent links (chemical bonding). Sometimes plasma methods are effective in the promotion of adhesion between parylene and polymeric surfaces but these techniques are not trivial to employ. The third surface, metal-oxide forming surfaces, generally possess a hydroxyl-terminated surface, M-OH, where M is a metal such as aluminum or chromium. This termination group has the ability to react with commercially available silanes such as A-174 (methacryloxypropyltrimethoxysilane), which is the common adhesion promoter for the parylene polymers.
The A-174 silane can be vapor delivered in situ or bonded via wet chemical baths. In all cases one half of the molecule binds to metal oxide forming surface through sol-gel chemistry (hydrolysis and condensation) and the other half co-polymerizes with parylene via a free radical addition
Radical polymerization
Free radical polymerization is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks. Free radicals can be formed via a number of different mechanisms usually involving separate initiator molecules...
reaction. In all cases the A-174 silane molecule 'lies down' on the surface and forms self-limited molecular layers of less than 1.0 nm. If thick layers are observed than the silane bath has started to 'polymerize' and a new bath should be started. Vapor phase silylation never yields more than a sub-monolayer of silane on the part being coated; and therefore this problem is circumvented.
History
Parylene development started in 1947, when Michael SzwarcMichael Szwarc
Michael Szwarc was a British and American polymer chemist who discovered and studied ionic living polymerization.- Biography :...
discovered the polymer as one of the thermal decomposition products of a common solvent p-xylene
P-Xylene
p-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents. The “p” stands for para, identifying the location of the methyl groups as across from one another....
at a temperatures exceeding 1000 °C. Szwarc first postulated the monomer to be para-xylylene which he confirmed by reacting the vapors with iodine and observing the para-xylylene di-iodide as the only product. The reaction yield was only a few percent, and a more efficient route was found later by William F. Gorham at Union Carbide. He deposited parylene films by the thermal decomposition of [2.2] paracyclophane at temperatures exceeding 550 °C and in vacuum below 1 Torr. This process did not require a solvent and resulted in chemically resistant films free from pinholes. Since the coating process takes place at ambient temperature in a mild vacuum, and because of parylene’s conformal properties, it has a wide variety of applications. Union Carbide commercialized a parylene coating system in 1965. Union Carbide went onto undertake research into the synthesis of numerous parylene precursors, including parylene AF-4, throughout the 1960s into the early 70's. There legacy lived on with NovaTran (now defunct) and now with Specialty Coating Systems. Very little innovation with the parylene polymers has been undertaken since via any of the commercial parylene companies. However, much university research utilizes the unique parylene polymers and their unique deposition process.
Characteristics and advantages
- Hydrophobic, chemically resistant coating with good barrier properties for inorganic and organic media, strong acids, caustic solutions, gases and water vapor
- Low leakage current and a low dielectric constant (average in-plane and out-of-plane: 2.67 parylene N and 2.5 parylene AF-4, SF, HT)
- A biostable, biocompatible coating; FDA approved for various applications
- Dense pinhole free, with thickness above 1.4 nm
- Coating without temperature load of the substrates as coating takes place at ambient temperature in the vacuum
- Highly corrosion resistant
- Completely homogeneousHomogeneous (chemistry)A substance that is uniform in composition is a definition of homogeneous. This is in contrast to a substance that is heterogeneous.The definition of homogeneous strongly depends on the context used. In Chemistry, a homogeneous suspension of material means that when dividing the volume in half, the...
surface - Oxidatively stable up to 350 °C (Parylene AF-4, SF, HT)
- Low intrinsic thin film stress due to its room temperature deposition
- Low coefficient of friction (AF-4, HT, SF)
- Very low permeabilityPermeability (fluid)Permeability in fluid mechanics and the earth sciences is a measure of the ability of a porous material to allow fluids to pass through it.- Units :...
to gases
Typical applications
Parylene films have been used in various applications, including- Hydrophobic coating (moisture barriers, e.g. for biomedical hoses)
- Barrier layers (e.g. for filter, diaphragms, valves)
- Microwave electronics
- Sensors in rough environment (e.g. automotive fuel/air sensors)
- Electronics for space travel and military
- Corrosion protection for metallic surfaces
- Reinforcement of micro-structures
- Protection of plastic, rubber, etc. from harmful environmental conditions
- Reduction of frictionFrictionFriction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
, e.g., for guiding catheters, acupuncture needles and Microelectromechanical systemsMicroelectromechanical systemsMicroelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
.

