Physisorption
Encyclopedia
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption
. The weak bonding of physisorption is due to the induced dipole moment
of a nonpolar adsorbate interacting with its own image charge in the polarizable solid.
. Even though the interaction energy is very weak (~10–100 meV), physisorption plays an important role in nature. For instance, the van der Waals attraction between surfaces and foot-hairs of gecko
s provides the remarkable ability to climb up vertical walls. In the point of view of molecular physics, the van der Waals attractive force originates from the charge fluctuations between two correlated bonding molecules or atoms, in other words, the mutually induced dipole moments. In comparison with chemisorption
, in which the electronic structure of bonding atoms or molecules is changed and covalent or ionic bonds form, physisorption, generally speaking, can only be observed in the environment of low temperature (thermal energy at room temperature ~26 meV) and the absence of the relatively strong chemisorptions.
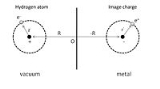
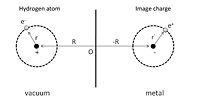 To give a simple illustration of physisorption, we can first consider an adsorbed hydrogen atom in front of a perfect conductor, as shown in Fig. 1. A nucleus with positive charge is located at R = (0, 0, Z), and the position coordinate of its electron, r = (x, y, z) is given with respect to the nucleus. The adsorption process can be viewed as the interaction between this hydrogen atom and its image charges of both the nucleus and electron in the conductor. As a result, the total electrostatic energy is the sum of attraction and repulsion terms:
To give a simple illustration of physisorption, we can first consider an adsorbed hydrogen atom in front of a perfect conductor, as shown in Fig. 1. A nucleus with positive charge is located at R = (0, 0, Z), and the position coordinate of its electron, r = (x, y, z) is given with respect to the nucleus. The adsorption process can be viewed as the interaction between this hydrogen atom and its image charges of both the nucleus and electron in the conductor. As a result, the total electrostatic energy is the sum of attraction and repulsion terms:
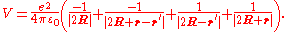
The first term is the attractive interaction of nucleus and its image charge, and the second term is due to the interaction of the electron and its image charge. The repulsive interaction is shown in the third and forth terms arising from the interaction of nucleus-image electron and electron-image nucleus, respectively.
By Taylor expansion in powers of |r| / |R|, this interaction energy can be further expressed as:
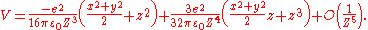
One can find from the first non-vanishing term that the physisorption potential depends on the distance Z between adsorbed atom and surface as Z−3, in contrast with the r−6 dependence of the molecular van der Waals
potential, where r is the distance between two dipoles.
binding energy can be analyzed by another simple physical picture: modeling the motion of an electron around its nucleus by a three-dimensional simple harmonic oscillator
with a potential energy Va:
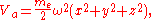
where me and ω are the mass and vibrational frequency of the electron, respectively.
As this atom approaches the surface of a metal and forms adsorption, this potential energy Va will be modified due to the image charges by additional potential terms which are quadratic in the displacements:
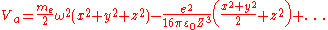 (from the Taylor expansion above.)
(from the Taylor expansion above.)
Assuming
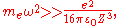
the potential is well approximated as
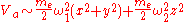 ,
,
where
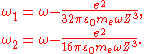
If one assumes that the electron is in the ground state, then the van der Waals binding energy is essentially the change of the zero-point energy:

This expression also shows the nature of the Z−3 dependence of the van der Waals interaction.
Furthermore by introducing the atomic polarizability
,
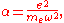
the van der Waals potential can be further simplified:
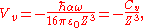
where
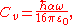
is the van der Waals constant which is related to the atomic polarizability.
Also, by expressing the fourth-order correction in the Taylor expansion above as (aCvZ0) / (Z4), where a is some constant, we can define Z0 as the position of the dynamical image plane and obtain
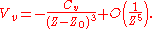
The origin of Z0 comes from the spilling of the electron wavefunction out of the surface. As a result, the position of image plane representing the reference for the space coordinate is different from the substrate surface itself and modified by Z0.
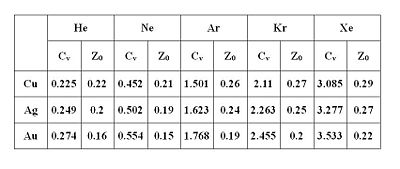
Table 1 shows the jellium
model calculation for van der Waals constant Cv and dynamical image plane Z0 of rare gas atoms on various metal surfaces. The increasing of Cv from He to Xe for all metal substrates is caused by the larger atomic polarizability
of the heavier rare gas atoms. For the position of the dynamical image plane, it decreases with increasing dielectric function and is typically on the order of 0.2 Å.
 Even though the van der Waals interaction is attractive, as the adsorbed atom moves closer to the surface the wavefunction of electron starts to overlap with that of the surface atoms. Further the energy of the system will increase due to the orthogonality of wavefunctions of the approaching atom and surface atoms.
Even though the van der Waals interaction is attractive, as the adsorbed atom moves closer to the surface the wavefunction of electron starts to overlap with that of the surface atoms. Further the energy of the system will increase due to the orthogonality of wavefunctions of the approaching atom and surface atoms.
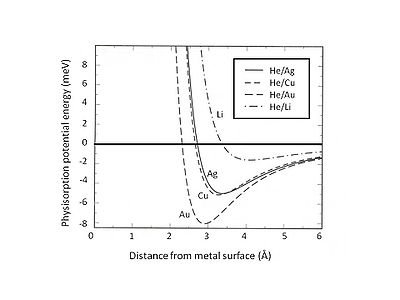
This Pauli exclusion and repulsion are particularly strong for atoms with closed valence shells that dominate the surface interaction. As a result, the minimum energy of physisorption must be found by the balance between the long-range van der Waals attraction and short-range Pauli repulsion. For instance, by separating the total interaction of physisorption into two contributions- a short-range term depicted by Hartree–Fock theory and a long-range van der Waals attraction, the equilibrium position of physisorption for rare gases adsorbed on jellium substrate can be determined. Fig. 2 shows the physisorption potential energy of He adsorbed on Ag, Cu, and Au substrates which are described by the jellium model with different densities of smear-out background positive charges. It can be found that the weak van der Waals interaction leads to shallow attractive energy wells (<10 meV). One of the experimental methods for exploring physisorption potential energy is the scattering process, for instance, inert gas atoms scattered from metal surfaces. Certain specific features of the interaction potential between scattered atoms and surface can be extracted by analyzing the experimentally determined angular distribution and cross sections of the scattered particles.
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
. The weak bonding of physisorption is due to the induced dipole moment
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
of a nonpolar adsorbate interacting with its own image charge in the polarizable solid.
Introduction
The fundamental interacting force of physisorption is caused by van der Waals forceVan der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
. Even though the interaction energy is very weak (~10–100 meV), physisorption plays an important role in nature. For instance, the van der Waals attraction between surfaces and foot-hairs of gecko
Gecko
Geckos are lizards belonging to the infraorder Gekkota, found in warm climates throughout the world. They range from 1.6 cm to 60 cm....
s provides the remarkable ability to climb up vertical walls. In the point of view of molecular physics, the van der Waals attractive force originates from the charge fluctuations between two correlated bonding molecules or atoms, in other words, the mutually induced dipole moments. In comparison with chemisorption
Chemisorption
Chemisorption is a sub-class of adsorption, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface...
, in which the electronic structure of bonding atoms or molecules is changed and covalent or ionic bonds form, physisorption, generally speaking, can only be observed in the environment of low temperature (thermal energy at room temperature ~26 meV) and the absence of the relatively strong chemisorptions.
Modeling by image charge

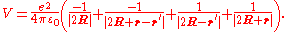
The first term is the attractive interaction of nucleus and its image charge, and the second term is due to the interaction of the electron and its image charge. The repulsive interaction is shown in the third and forth terms arising from the interaction of nucleus-image electron and electron-image nucleus, respectively.
By Taylor expansion in powers of |r| / |R|, this interaction energy can be further expressed as:
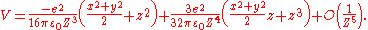
One can find from the first non-vanishing term that the physisorption potential depends on the distance Z between adsorbed atom and surface as Z−3, in contrast with the r−6 dependence of the molecular van der Waals
Van der Waals
-People:* Fransje van der Waals , Dutch medical physician* Johannes Diderik van der Waals , Dutch physicist-Physics:* the Van der Waals force, named after the physicist* the Van der Waals equation, named after the physicist...
potential, where r is the distance between two dipoles.
Modeling by quantum-mechanical oscillator
The van der WaalsVan der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
binding energy can be analyzed by another simple physical picture: modeling the motion of an electron around its nucleus by a three-dimensional simple harmonic oscillator
Harmonic oscillator
In classical mechanics, a harmonic oscillator is a system that, when displaced from its equilibrium position, experiences a restoring force, F, proportional to the displacement, x: \vec F = -k \vec x \, where k is a positive constant....
with a potential energy Va:
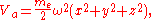
where me and ω are the mass and vibrational frequency of the electron, respectively.
As this atom approaches the surface of a metal and forms adsorption, this potential energy Va will be modified due to the image charges by additional potential terms which are quadratic in the displacements:
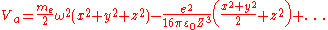 (from the Taylor expansion above.)
(from the Taylor expansion above.)Assuming
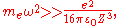
the potential is well approximated as
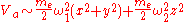 ,
,where
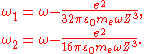
If one assumes that the electron is in the ground state, then the van der Waals binding energy is essentially the change of the zero-point energy:

This expression also shows the nature of the Z−3 dependence of the van der Waals interaction.
Furthermore by introducing the atomic polarizability
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
,
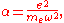
the van der Waals potential can be further simplified:
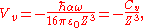
where
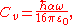
is the van der Waals constant which is related to the atomic polarizability.
Also, by expressing the fourth-order correction in the Taylor expansion above as (aCvZ0) / (Z4), where a is some constant, we can define Z0 as the position of the dynamical image plane and obtain

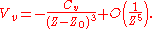
The origin of Z0 comes from the spilling of the electron wavefunction out of the surface. As a result, the position of image plane representing the reference for the space coordinate is different from the substrate surface itself and modified by Z0.
Table 1 shows the jellium
Jellium
Jellium, also known as the uniform electron gas or homogeneous electron gas , is a quantum mechanical model of interacting electrons in a solid where the positive charges are assumed to be uniformly distributed in space whence the electron densityis a uniform quantity as well in space...
model calculation for van der Waals constant Cv and dynamical image plane Z0 of rare gas atoms on various metal surfaces. The increasing of Cv from He to Xe for all metal substrates is caused by the larger atomic polarizability
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
of the heavier rare gas atoms. For the position of the dynamical image plane, it decreases with increasing dielectric function and is typically on the order of 0.2 Å.
Physisorption potential

This Pauli exclusion and repulsion are particularly strong for atoms with closed valence shells that dominate the surface interaction. As a result, the minimum energy of physisorption must be found by the balance between the long-range van der Waals attraction and short-range Pauli repulsion. For instance, by separating the total interaction of physisorption into two contributions- a short-range term depicted by Hartree–Fock theory and a long-range van der Waals attraction, the equilibrium position of physisorption for rare gases adsorbed on jellium substrate can be determined. Fig. 2 shows the physisorption potential energy of He adsorbed on Ag, Cu, and Au substrates which are described by the jellium model with different densities of smear-out background positive charges. It can be found that the weak van der Waals interaction leads to shallow attractive energy wells (<10 meV). One of the experimental methods for exploring physisorption potential energy is the scattering process, for instance, inert gas atoms scattered from metal surfaces. Certain specific features of the interaction potential between scattered atoms and surface can be extracted by analyzing the experimentally determined angular distribution and cross sections of the scattered particles.
Comparison with chemisorption
- Physisorption is a general phenomenon and occurs in any solid/fluid or solid/gas system. ChemisorptionChemisorptionChemisorption is a sub-class of adsorption, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface...
is characterized by chemical specificity. - In physiorption, perturbation of the electronic states of adsorbent and adsorbate is minimal. For chemisorption, changes in the electronic states may be detectable by suitable physical means.
- Typical binding energy of physisorption is about 10–100 meV. Chemisorption usually forms bonding with energy of 1–10 eV.
- The elementary step in physisorption from a gas phase does not involve an activation energy. Chemisorption often involves an activation energy.
- For physisorption, under appropriate conditions, gas phase molecules can form multilayer adsorption. In chemisorption, molecules are adsorbed on the surface by valence bonds and only form monolayer adsorption.

