
Chemisorption
Encyclopedia
Chemisorption is a sub-class of adsorption
, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface (e.g. corrosion, metallic oxidation). The strong interaction between the adsorbate and the substrate
surface
creates new types of electronic bonds
- ionic
or covalent
, depending on the reactive chemical species involved.
Note: not to be confused with physical adsorption
which leaves the chemical species of the adsorbate and surface intact. It is conventionally accepted that the energetic threshold separating the binding energy
of "adsorption" from that of "chemisorption" is about 0.5 eV per atom
or molecule
.
It is characterised by:
Due to specificity, the nature of chemisorption can greatly differ from system to system, depending on the chemical identity and the surface structure.
reactions. The process of chemisorption is actually pivotal to the role of heterogeneous catalysis
where the catalyst is in a solid phase—particularly transition metal
catalysts. In many cases the chemical reagents will both bind to the catalytic surface. The chemical bond
s then form and draw electron
s away from the chemisorption bonds. The molecule then desorbs and is free to leave the surface.
s (SAMs) are often formed by chemisorbing thiol
s (RS-H) onto gold
surfaces forming Au-SR bonds.
O2 on carbon at high temperatures.
Research is ongoing on the adsorption of hydrogen onto carbon nanotube
s with the aim of producing a fuel cell
that can eventually replace our dependence on fossil fuels.
. If it elastically collides with the surface, then it would return to the bulk gas. If it loses enough momentum
through an inelastic collision
, then it “sticks” onto the surface, forming a precursor state bonded to the surface by weak forces, similar to physisorption. The particle diffuses on the surface until it finds a deep chemisorption potential well. Then it reacts with the surface or simply desorbs after enough energy and time.
The reaction with the surface is dependent on the chemical species involved. Applying Gibbs free energy
equation for reactions:
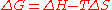
General thermodynamics
states that for spontaneous reactions, the change in free energy should be negative. Since a free particle is restrained to a surface, and unless the surface atom is highly mobile, entropy is lowered. This means that the enthalpy
term must be negative, implying an exothermic reaction
.
Figure 1 is a graph of physisorption and chemisorption energy curves of tungsten
and oxygen
. Physisorption is given as a Lennard-Jones potential
and chemisorption is given as a Morse potential
. There exists a point of crossover between the physisorption and chemisorption, meaning a point of transfer. It can occur above or below the zero-energy line (with a difference in the Morse potential, a), representing an activation energy
requirement or lack of. Most simple gases on clean metal surfaces lack the activation energy requirement.
However, chemisorption is very difficult to theorize. A multidimensional potential energy surface
(PES) derived from effective medium theory
is used to describe the effect of the surface on absorption, but only certain parts of it are used depending on what is to be studied. A simple example of a PES, which takes the total of the energy as a function of location:
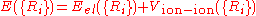
where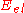 is the energy eigenvalue of the Schrödinger equation
is the energy eigenvalue of the Schrödinger equation
for the electronic degrees of freedom and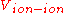 is the ion interactions. This expression is without translational energy, rotational energy
is the ion interactions. This expression is without translational energy, rotational energy
, vibrational excitations, and other such considerations.
There exist several models to describe surface reactions: the Langmuir-Hinschelwood mechanism
in which both reacting species are adsorbed, and the Eley-Rideal mechanism
in which one is adsorbed and the other reacts with it.
Real systems have many irregularities, making theoretical calculations more difficult:
Compared to physisorption where adsorbates are simply sitting on the surface, the adsorbates can change the surface, along with its structure. The structure can go through relaxation, where the first few layers change interplanar distances without changing the surface structure, or reconstruction where the surface structure is changed.
For example oxygen can form very strong bonds (~4 eV) with metals, such as Cu(110). This comes with the breaking apart of surface bonds in forming surface-adsorbate bonds. A large restructuring occurs by missing row as seen in Figure 2.
of diatomic gas molecules, such as hydrogen
, oxygen
, and nitrogen
. One model used to describe the process is precursor-mediation. The absorbed molecule is adsorbed onto a surface into a precursor state. The molecule then diffuses across the surface to the chemisorption sites. They break the molecular bond in favor of new bonds to the surface. The energy to overcome the activation potential of dissociation usually comes from the translational energy and vibrational energy.
And example is the hydrogen and copper
system, one that has been studied many times over. It has a large activation energy of .35 - .85 eV. The vibrational excitation of the hydrogen molecule promotes dissociation on low index surfaces of copper.
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface (e.g. corrosion, metallic oxidation). The strong interaction between the adsorbate and the substrate
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
surface
Surface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
creates new types of electronic bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
- ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
or covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
, depending on the reactive chemical species involved.
Note: not to be confused with physical adsorption
Physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption...
which leaves the chemical species of the adsorbate and surface intact. It is conventionally accepted that the energetic threshold separating the binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
of "adsorption" from that of "chemisorption" is about 0.5 eV per atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
.
It is characterised by:
- An activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for the chemical reaction that takes place only in a monolayerMonolayer- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail... - A high enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change/ temperature gain, indicating an exothermicExothermicIn thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
chemical reaction: -20 kJ/mol >ΔH> -200 kJ/mol
Due to specificity, the nature of chemisorption can greatly differ from system to system, depending on the chemical identity and the surface structure.
Uses
The main way in which most chemists utilise the effect of chemisorption is in catalysedCatalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
reactions. The process of chemisorption is actually pivotal to the role of heterogeneous catalysis
Heterogeneous catalysis
In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts...
where the catalyst is in a solid phase—particularly transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
catalysts. In many cases the chemical reagents will both bind to the catalytic surface. The chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s then form and draw electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s away from the chemisorption bonds. The molecule then desorbs and is free to leave the surface.
Examples
Self-assembled monolayerSelf-assembled monolayer
A self assembled monolayer is an organized layer of amphiphilic molecules in which one end of the molecule, the “head group” shows a specific, reversible affinity for a substrate...
s (SAMs) are often formed by chemisorbing thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s (RS-H) onto gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
surfaces forming Au-SR bonds.
O2 on carbon at high temperatures.
Research is ongoing on the adsorption of hydrogen onto carbon nanotube
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
s with the aim of producing a fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
that can eventually replace our dependence on fossil fuels.
Adsorption Kinetics
As an instance of adsorption, chemisorption follows the adsorption process. The first stage is for the adsorbate particle to come into contact with the surface. The particle needs to be trapped onto the surface by not possessing enough energy to leave the gas-surface potential wellPotential well
A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy because it is captured in the local minimum of a potential well...
. If it elastically collides with the surface, then it would return to the bulk gas. If it loses enough momentum
Momentum
In classical mechanics, linear momentum or translational momentum is the product of the mass and velocity of an object...
through an inelastic collision
Inelastic collision
An inelastic collision, in contrast to an elastic collision, is a collision in which kinetic energy is not conserved.In collisions of macroscopic bodies, some kinetic energy is turned into vibrational energy of the atoms, causing a heating effect, and the bodies are deformed.The molecules of a gas...
, then it “sticks” onto the surface, forming a precursor state bonded to the surface by weak forces, similar to physisorption. The particle diffuses on the surface until it finds a deep chemisorption potential well. Then it reacts with the surface or simply desorbs after enough energy and time.
The reaction with the surface is dependent on the chemical species involved. Applying Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
equation for reactions:
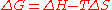
General thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
states that for spontaneous reactions, the change in free energy should be negative. Since a free particle is restrained to a surface, and unless the surface atom is highly mobile, entropy is lowered. This means that the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
term must be negative, implying an exothermic reaction
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
.
Figure 1 is a graph of physisorption and chemisorption energy curves of tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. Physisorption is given as a Lennard-Jones potential
Lennard-Jones potential
The Lennard-Jones potential is a mathematically simple model that approximates the interaction between a pair of neutral atoms or molecules. A form of the potential was first proposed in 1924 by John Lennard-Jones...
and chemisorption is given as a Morse potential
Morse potential
The Morse potential, named after physicist Philip M. Morse, is a convenient model for the potential energy of a diatomic molecule. It is a better approximation for the vibrational structure of the molecule than the quantum harmonic oscillator because it explicitly includes the effects of bond...
. There exists a point of crossover between the physisorption and chemisorption, meaning a point of transfer. It can occur above or below the zero-energy line (with a difference in the Morse potential, a), representing an activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
requirement or lack of. Most simple gases on clean metal surfaces lack the activation energy requirement.
Modeling
For experimental setups of chemisorption, the amount of adsorption of a particular system is quantified by a sticking probability value.However, chemisorption is very difficult to theorize. A multidimensional potential energy surface
Potential energy surface
A potential energy surface is generally used within the adiabatic or Born–Oppenheimer approximation in quantum mechanics and statistical mechanics to model chemical reactions and interactions in simple chemical and physical systems...
(PES) derived from effective medium theory
Effective Medium Approximations
Effective medium approximations or effective medium theory are physical models that describe the macroscopic properties of a medium based on the properties and the relative fractions of its components...
is used to describe the effect of the surface on absorption, but only certain parts of it are used depending on what is to be studied. A simple example of a PES, which takes the total of the energy as a function of location:
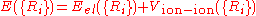
where
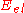 is the energy eigenvalue of the Schrödinger equation
is the energy eigenvalue of the Schrödinger equationSchrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
for the electronic degrees of freedom and
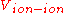 is the ion interactions. This expression is without translational energy, rotational energy
is the ion interactions. This expression is without translational energy, rotational energyRotational energy
The rotational energy or angular kinetic energy is the kinetic energy due to the rotation of an object and is part of its total kinetic energy...
, vibrational excitations, and other such considerations.
There exist several models to describe surface reactions: the Langmuir-Hinschelwood mechanism
Langmuir adsorption model
The Langmuir adsorption model is the most common model used to quantify the amount of adsorbate adsorbed on an adsorbent as a function of partial pressure or concentration at a given temperature. It considers adsorption of an ideal gas onto an idealized surface...
in which both reacting species are adsorbed, and the Eley-Rideal mechanism
Reactions on surfaces
By reactions on surfaces it is understood reactions in which at least one of the steps of the reaction mechanism is the adsorption of one or more reactants...
in which one is adsorbed and the other reacts with it.
Real systems have many irregularities, making theoretical calculations more difficult:
- Solid surfaces are not necessarily at equilibrium.
- They may be perturbed and irregular, defects and such.
- Distribution of adsorption energies and odd adsorption sites.
- Bonds formed between the adsorbates.
Compared to physisorption where adsorbates are simply sitting on the surface, the adsorbates can change the surface, along with its structure. The structure can go through relaxation, where the first few layers change interplanar distances without changing the surface structure, or reconstruction where the surface structure is changed.
For example oxygen can form very strong bonds (~4 eV) with metals, such as Cu(110). This comes with the breaking apart of surface bonds in forming surface-adsorbate bonds. A large restructuring occurs by missing row as seen in Figure 2.
Dissociation Chemisorption
A particular brand of gas-surface chemisorption is the dissociationDissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
of diatomic gas molecules, such as hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. One model used to describe the process is precursor-mediation. The absorbed molecule is adsorbed onto a surface into a precursor state. The molecule then diffuses across the surface to the chemisorption sites. They break the molecular bond in favor of new bonds to the surface. The energy to overcome the activation potential of dissociation usually comes from the translational energy and vibrational energy.
And example is the hydrogen and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
system, one that has been studied many times over. It has a large activation energy of .35 - .85 eV. The vibrational excitation of the hydrogen molecule promotes dissociation on low index surfaces of copper.

