Penicillin binding proteins
Encyclopedia
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s that are characterized by their affinity for and binding of penicillin
Penicillin
Penicillin is a group of antibiotics derived from Penicillium fungi. They include penicillin G, procaine penicillin, benzathine penicillin, and penicillin V....
. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotic
Beta-lactam antibiotic
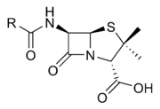
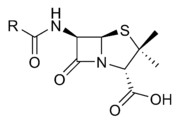
β-Lactam antibiotics are a broad class of antibiotics, consisting of all antibiotic agents that contains a β-lactam nucleus in its molecular structure. This includes penicillin derivatives , cephalosporins , monobactams, and carbapenems...
s (except for tabtoxinine-β-lactam, which inhibits glutamine synthetase
Glutamine synthetase
Glutamine synthetase is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:Glutamate + ATP + NH3 → Glutamine + ADP + phosphate...
) bind to PBP to prevent the bacterium from constructing a cell wall
Cell wall
The cell wall is the tough, usually flexible but sometimes fairly rigid layer that surrounds some types of cells. It is located outside the cell membrane and provides these cells with structural support and protection, and also acts as a filtering mechanism. A major function of the cell wall is to...
.
Diversity
There are a large number of PBPs, usually several in each organism, and they are found as both membrane-bound and cytoplasmic proteins. For example, Spratt (1977) reports that six different PBPs are routinely detected in all strains of E. coliEscherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
ranging in molecular weight from 40,000 to 91,000. The different PBPs occur in different numbers per cell and have varied affinities for penicillin (see appendix). The PBPs are usually broadly classified into high-molecular-weight (HMW) and low-molecular-weight (LMW) categories.
Function
PBPs are all involved in the final stages of the synthesis of peptidoglycanPeptidoglycan
Peptidoglycan, also known as murein, is a polymer consisting of sugars and amino acids that forms a mesh-like layer outside the plasma membrane of bacteria , forming the cell wall. The sugar component consists of alternating residues of β- linked N-acetylglucosamine and N-acetylmuramic acid...
, which is the major component of bacterial cell walls. Bacterial cell wall synthesis is essential to growth, cell division (thus reproduction) and maintaining the cellular structure in bacteria. Inhibition of PBPs leads to irregularities in cell wall structure such as elongation, lesions, loss of selective permeability, and eventual cell death and lysis
Lysis
Lysis refers to the breaking down of a cell, often by viral, enzymic, or osmotic mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a "lysate"....
.
PBPs have been shown to catalyze a number of reactions involved in the process of synthesizing cross-linked peptidoglycan from lipid intermediates and mediating the removal of D-alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
from the precursor of peptidoglycan. Purified enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s have been shown to catalyze the following reactions: D-alanine carboxypeptidase, peptidoglycan transpeptidase, and peptidoglycan endopeptidase. In all bacteria that have been studied, enzymes have been shown to catalyze more than one of the above reactions. The enzyme has a penicillin-insensitive transglycosylase N-terminal
N-terminal end
The N-terminus refers to the start of a protein or polypeptide terminated by an amino acid with a free amine group . The convention for writing peptide sequences is to put the N-terminus on the left and write the sequence from N- to C-terminus...
domain (involved in formation of linear glycan strands) and a penicillin-sensitive transpeptidase C-terminal
C-terminal end
The C-terminus is the end of an amino acid chain , terminated by a free carboxyl group . When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus...
domain (involved in cross-linking of the peptide subunits) and the serine at the active site is conserved in all members of the PBP family.
Antibiotics
PBPs bind β-lactam antibiotics because they are similar in chemical structure to the modular pieces that form the peptidoglycan. When they bind to penicillin, the β-lactam amide bond is ruptured to form a covalent bond with the serine residue at the PBPs active site. This is an irreversible reaction and inactivates the enzyme.There has been a great deal of research into PBPs because of their role in antibiotics and resistance. Bacterial cell wall synthesis and the role of PBPs in its synthesis is a very good target for drugs of selective toxicity because the metabolic pathways and enzymes are unique to bacteria. Resistance to antibiotics has come about through overproduction of PBPs and formation of PBPs that have low affinity for penicillins (among other mechanisms such as lactamase production). Research on PBPs has led to the discovery of new semi-synthetic β-lactams, wherein altering the side-chains on the original penicillin molecule has increased the affinity of PBPs for penicillin, and, thus, increased effectiveness in bacteria with developing resistance.
Presence of the protein Penicillin binding protein 2A (PBP2A) is responsible for the antibiotic resistance seen in methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus is a bacterium responsible for several difficult-to-treat infections in humans. It is also called multidrug-resistant Staphylococcus aureus and oxacillin-resistant Staphylococcus aureus...
(MRSA).
The β-lactam ring is a structure common to all β-lactam antibiotics.

