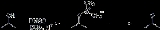
Pfitzner-Moffatt oxidation
Encyclopedia
The Pfitzner–Moffatt oxidation, sometimes referred to as simply the Moffatt oxidation, is a chemical reaction
which describes the oxidation
of primary and secondary alcohols by dimethyl sulfoxide
(DMSO) activated with a carbodiimide, such as dicyclohexylcarbodiimide
(DCC). The resulting alkoxysulfonium ylide
rearranges to generate aldehyde
s and ketone
s, respectively.
This reaction has been largely abandoned for the Swern oxidation
, which gives higher yields with fewer side products. The Moffatt oxidation yields urea biproducts that are often difficult to remove.
Several reviews have been published.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
which describes the oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of primary and secondary alcohols by dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
(DMSO) activated with a carbodiimide, such as dicyclohexylcarbodiimide
Dicyclohexylcarbodiimide
N,N-Dicyclohexylcarbodiimide is an organic compound with chemical formula C13H22N2 whose primary use is to couple amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material...
(DCC). The resulting alkoxysulfonium ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
rearranges to generate aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, respectively.
This reaction has been largely abandoned for the Swern oxidation
Swern oxidation
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide and an organic base, such as triethylamine...
, which gives higher yields with fewer side products. The Moffatt oxidation yields urea biproducts that are often difficult to remove.
Several reviews have been published.

