
Pharmacophore
Encyclopedia
A pharmacophore is an abstract description of molecular features which are necessary for molecular recognition
of a ligand by a biological macromolecule
.
The IUPAC defines a pharmacophore to be "an ensemble of steric and electronic features that is necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger (or block) its biological response". In simple English, a pharmacophore is the portion of a ligand molecule which binds to the receptor site. The pharmacophore of a ligand may be localised in a particular area of the ligand molecule or it may be spread across different areas of the ligand molecule. An example of this is the neuromuscular blocking agent, tubocurarine
. The quaternary nitrogens present in the tubocurarine molecule are believed to make up its pharmacophore.
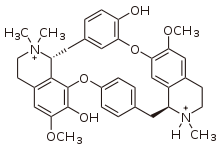 As can be seen, the quaternary nitrogens are not localised to a particular area of the molecule, but are rather dispersed across molecule.
As can be seen, the quaternary nitrogens are not localised to a particular area of the molecule, but are rather dispersed across molecule.
Historically, pharmacophores were established by Lemont Kier
, who first mentions the concept in 1967.
and uses the term in a publication in 1971.
The development of the concept is often erroneously accredited to Paul Ehrlich
. However neither the alleged source nor any of his other works mention the term "pharmacophore" or make use of the concept.
In modern computational chemistry
, pharmacophores are used to define the essential features of one or more molecules with the same biological activity
. A database of diverse chemical compound
s can then be searched for more molecules which share the same features located a similar distance apart from each other.
Typical pharmacophore features are for where a molecule is hydrophobic, aromatic, a hydrogen bond
acceptor, a hydrogen bond
donor, a cation, or an anion. The features need to match different chemical groups with similar properties, in order to identify novel ligands. Ligands receptor interactions are typically “polar positive”, “polar negative” or “hydrophobic”. A well-defined pharmacophore model includes both hydrophobic volumes and hydrogen bond vectors.
Molecular recognition
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, electrostatic and/or electromagnetic effects...
of a ligand by a biological macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
.
The IUPAC defines a pharmacophore to be "an ensemble of steric and electronic features that is necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger (or block) its biological response". In simple English, a pharmacophore is the portion of a ligand molecule which binds to the receptor site. The pharmacophore of a ligand may be localised in a particular area of the ligand molecule or it may be spread across different areas of the ligand molecule. An example of this is the neuromuscular blocking agent, tubocurarine
Tubocurarine
Tubocurarine is a neuromuscular-blocking drug or skeletal muscle relaxant in the category of non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to provide skeletal muscle relaxation during surgery or mechanical ventilation...
. The quaternary nitrogens present in the tubocurarine molecule are believed to make up its pharmacophore.

Historically, pharmacophores were established by Lemont Kier
Lemont Kier
Lemont Kier is an American chemist and leader in the field of Drug Design and Medicinal Chemistry. He is the recipient of the American Association of Pharmaceutical Scientists 2008 Research Achievement Award in Drug Development and Discovery. He obtained his PhD in Medicinal Chemistry at the...
, who first mentions the concept in 1967.
and uses the term in a publication in 1971.
The development of the concept is often erroneously accredited to Paul Ehrlich
Paul Ehrlich
Paul Ehrlich was a German scientist in the fields of hematology, immunology, and chemotherapy, and Nobel laureate. He is noted for curing syphilis and for his research in autoimmunity, calling it "horror autotoxicus"...
. However neither the alleged source nor any of his other works mention the term "pharmacophore" or make use of the concept.
In modern computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
, pharmacophores are used to define the essential features of one or more molecules with the same biological activity
Biological activity
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or pharmacophore but can be modified by the other...
. A database of diverse chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s can then be searched for more molecules which share the same features located a similar distance apart from each other.
Typical pharmacophore features are for where a molecule is hydrophobic, aromatic, a hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
acceptor, a hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
donor, a cation, or an anion. The features need to match different chemical groups with similar properties, in order to identify novel ligands. Ligands receptor interactions are typically “polar positive”, “polar negative” or “hydrophobic”. A well-defined pharmacophore model includes both hydrophobic volumes and hydrogen bond vectors.
See also
- CheminformaticsCheminformaticsCheminformatics is the use of computer and informational techniques, applied to a range of problems in the field of chemistry. These in silico techniques are used in pharmaceutical companies in the process of drug discovery...
- Molecule miningMolecule miningThis page describes mining for molecules. Since molecules may be represented by molecular graphs this is strongly related to graph mining and structured data mining. The main problem is how to represent molecules while discriminating the data instances...
- Pharmaceutical companyPharmaceutical companyThe pharmaceutical industry develops, produces, and markets drugs licensed for use as medications. Pharmaceutical companies are allowed to deal in generic and/or brand medications and medical devices...
- QSAR
- in silicoIn silicoIn silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...

