
Phenylpropanoid
Encyclopedia
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acid
phenylalanine
. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid
, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogen
s, and mediate plant-pollinator
interactions as floral pigments and scent compounds.
by the action of the enzyme
phenylalanine ammonia-lyase
(PAL). A series of enzymatic hydroxylation
s and methylation
s leads to coumaric acid
, caffeic acid
, ferulic acid
, 5-hydroxyferulic acid, and sinapic acid. Conversion of these acids to their corresponding ester
s produces some of the volatile components of herb and flower fragrances, which serve many functions such as attracting pollinator
s. Ethyl cinnamate
is a common example.
functional groups in the cinnamic acids provides the corresponding aldehydes, such as cinnamaldehyde
. Further reduction provides monolignol
s including coumaryl alcohol, coniferyl alcohol
, and sinapyl alcohol
, which vary only in their degree of methoxylation
. The monolignols are monomers that are polymer
ized to generate various forms of lignin
and suberin
, which are used as a structural component of plant cell walls.
The phenylpropene
s, including eugenol
, chavicol
, safrole
and estragole
, are also derived from the monolignols. These compounds are the primary constituents of various essential oil
s.
in the 4-position leads to p-coumaric acid
, which can be further modified into hydroxylated derivatives such as umbelliferone
. Another use of p-coumaric acid via its thioester
with coenzyme A
, i.e. 4-coumaroyl-CoA, is the production of chalcones. This is achieved with the addition of 3 malonyl-CoA
molecules and their cyclization into a second phenyl group. Chalcones are the precursors of all flavonoid
s, a diverse class of phytochemical
s.
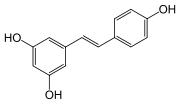 Stilbenoids, such as resveratrol
Stilbenoids, such as resveratrol
, are hydroxylated derivatives of stilbene
. They are formed through an alternative cyclization of cinammoyl-CoA or 4-coumaroyl-CoA.
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid
Cinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogen
Pathogen
A pathogen gignomai "I give birth to") or infectious agent — colloquially, a germ — is a microbe or microorganism such as a virus, bacterium, prion, or fungus that causes disease in its animal or plant host...
s, and mediate plant-pollinator
Pollinator
A pollinator is the biotic agent that moves pollen from the male anthers of a flower to the female stigma of a flower to accomplish fertilization or syngamy of the female gamete in the ovule of the flower by the male gamete from the pollen grain...
interactions as floral pigments and scent compounds.
Hydroxycinnamic acids
Phenylalanine is first converted to cinnamic acidCinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
by the action of the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
phenylalanine ammonia-lyase
Phenylalanine ammonia-lyase
In enzymology, a phenylalanine ammonia-lyase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, L-phenylalanine, and two products, trans-cinnamic acid and ammonia....
(PAL). A series of enzymatic hydroxylation
Hydroxylation
Hydroxylation is a chemical process that introduces a hydroxyl group into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air...
s and methylation
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
s leads to coumaric acid
Coumaric acid
Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers, o-coumaric acid, m-coumaric acid, and p-coumaric acid, that differ by the position of the hydroxy substitution of the phenyl group...
, caffeic acid
Caffeic acid
Caffeic acid is a hydroxycinnamic acid, a naturally occurring organic compound. This yellow solid consists of both phenolic and acrylic functional groups...
, ferulic acid
Ferulic acid
Ferulic acid is a hydroxycinnamic acid, a type of organic compound. It is an abundant phenolic phytochemical found in plant cell wall components such as arabinoxylans as covalent side chains. It is related to trans-cinnamic acid. As a component of lignin, ferulic acid is a precursor in the...
, 5-hydroxyferulic acid, and sinapic acid. Conversion of these acids to their corresponding ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s produces some of the volatile components of herb and flower fragrances, which serve many functions such as attracting pollinator
Pollinator
A pollinator is the biotic agent that moves pollen from the male anthers of a flower to the female stigma of a flower to accomplish fertilization or syngamy of the female gamete in the ovule of the flower by the male gamete from the pollen grain...
s. Ethyl cinnamate
Ethyl cinnamate
Ethyl cinnamate is the ester of cinnamic acid and ethanol. It is present in the essential oil of cinnamon. Pure ethyl cinnamate has a "fruity and balsamic odor, reminiscent of cinnamon with an amber note"....
is a common example.
Cinnamic aldehydes and monolignols
Reduction of the carboxylic acidCarboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
functional groups in the cinnamic acids provides the corresponding aldehydes, such as cinnamaldehyde
Cinnamaldehyde
Cinnamaldehyde is the organic compound that gives cinnamon its flavor and odor. This pale yellow viscous liquid occurs naturally in the bark of cinnamon trees and other species of the genus Cinnamomum...
. Further reduction provides monolignol
Monolignol
Monolignols are phytochemicals acting as source materials for biosynthesis of both lignans and lignin. The starting material for production of monolignols is the amino acid phenylalanine. The first reactions in the biosynthesis are shared with the phenylpropanoid pathway, and monolignols are...
s including coumaryl alcohol, coniferyl alcohol
Coniferyl alcohol
Coniferyl alcohol is an organic compound. This colourless crystalline solid is a phytochemical, one of the monolignols. It is synthethized via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans...
, and sinapyl alcohol
Sinapyl alcohol
Sinapyl alcohol is an organic compound derived from cinnamic acid. This phytochemical is one of the monolignols. It is biosynthetized via the phenylpropanoid biochemical pathway, its immediate precursor being sinapaldehyde. Sinapyl alcohol is a precursor to lignin or lignans. It is also a...
, which vary only in their degree of methoxylation
Methoxy
In chemistry , methoxy refers to the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula O–CH3.The word is used in organic nomenclature usually to describe an ether...
. The monolignols are monomers that are polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
ized to generate various forms of lignin
Lignin
Lignin or lignen is a complex chemical compound most commonly derived from wood, and an integral part of the secondary cell walls of plants and some algae. The term was introduced in 1819 by de Candolle and is derived from the Latin word lignum, meaning wood...
and suberin
Suberin
Suberin is a waxy substance found in higher plants. Suberin is a main constituent of cork, and is named after the Cork Oak, Quercus suber.-Anatomy and physiology:...
, which are used as a structural component of plant cell walls.
The phenylpropene
Phenylpropene
Phenylpropenes, propenylphenols or allylbenzenes are a class of phenylpropanoids, a type of polyphenols.The phenylpropenes, including eugenol, chavicol, safrole and estragole, are derived from the monolignols. These compounds are the primary constituents of various essential oils....
s, including eugenol
Eugenol
Eugenol is a phenylpropene, an allyl chain-substituted guaiacol. Eugenol is a member of the phenylpropanoids class of chemical compounds. It is a clear to pale yellow oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil and bay leaf. It is slightly...
, chavicol
Chavicol
Chavicol, or p-allylphenol, is a natural phenylpropene, a type of organic compound. Its chemical structure consists of a benzene ring substituted with a hydroxy group and a propenyl group. It is a colorless liquid found together with terpenes in betel oil. It is miscible with alcohol, ether, and...
, safrole
Safrole
Safrole, also known as shikimol, is a phenylpropene. It is a colorless or slightly yellow oily liquid. It is typically extracted from the root-bark or the fruit of sassafras plants in the form of sassafras oil , or synthesized from other related methylenedioxy...
and estragole
Estragole
Estragole is a phenylpropene, a natural organic compound. Its chemical structure consists of a benzene ring substituted with a methoxy group and a propenyl group. Estragole is a double-bond isomer of anethole. It is a colorless to pale yellow liquid...
, are also derived from the monolignols. These compounds are the primary constituents of various essential oil
Essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants. Essential oils are also known as volatile oils, ethereal oils or aetherolea, or simply as the "oil of" the plant from which they were extracted, such as oil of clove...
s.
Coumarins and flavonoids
Hydroxylation of cinnamic acidCinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
in the 4-position leads to p-coumaric acid
Coumaric acid
Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers, o-coumaric acid, m-coumaric acid, and p-coumaric acid, that differ by the position of the hydroxy substitution of the phenyl group...
, which can be further modified into hydroxylated derivatives such as umbelliferone
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and beta-umbelliferone, is a widespread natural product of the coumarin family. It occurs in many familiar plants from the Apiaceae family such as carrot, coriander and garden angelica, as well plants from other families such...
. Another use of p-coumaric acid via its thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
with coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
, i.e. 4-coumaroyl-CoA, is the production of chalcones. This is achieved with the addition of 3 malonyl-CoA
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative.-Functions:It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis....
molecules and their cyclization into a second phenyl group. Chalcones are the precursors of all flavonoid
Flavonoid
Flavonoids , are a class of plant secondary metabolites....
s, a diverse class of phytochemical
Phytochemical
Phytochemicals are biologically active chemical compounds that occur naturally in plants . Phytochemicals are the molecules responsible for the color and organoleptic properties . For example, the deep purple color of blueberries and the smell of garlic...
s.
Stilbenoids

Resveratrol
Resveratrol is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants when under attack by pathogens such as bacteria or fungi....
, are hydroxylated derivatives of stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
. They are formed through an alternative cyclization of cinammoyl-CoA or 4-coumaroyl-CoA.

