
Photosystem II
Encyclopedia
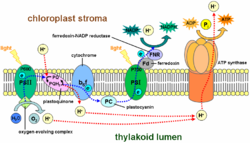
Light-dependent reactions
The 'light-dependent reactions', or light reactions, are the first stage of photosynthesis, the process by which plants capture and store energy from sunlight. In this process, light energy is converted into chemical energy, in the form of the energy-carrying molecules ATP and NADPH...
. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. The enzyme uses photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
to reduce plastoquinone
Plastoquinone
Plastoquinone is a quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. Plastoquinone is reduced , forming plastoquinol...
to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen. By obtaining these electrons from water, photosystem II provides the electrons for all of photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
to generate ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
. The energized electrons transferred to plastoquinone are ultimately used to reduce to NADPH or are used in Cyclic Photophosphorylation.
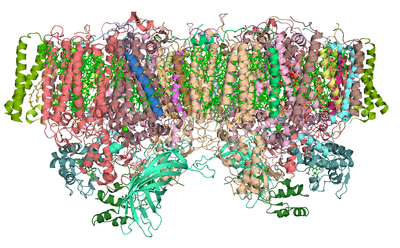
Structure
Photosystem II (of cyanobacteria and green plants) is composed of 20 subunits as well as other accessory, light-harvesting proteins. Each photosystem II contains at least 99 cofactors: 35 chlorophyll a, 12 beta-carotene, two pheophytin, three plastoquinone, two heme, bicarbonate, 25 lipid, and seven n-dodecyl-beta-D-maltoside detergent molecules, the six components of the cluster (including chloride ion), and one and two putative ion per monomer. There are several crystal structures of photosystem II. The PDBProtein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes for this protein are , (3BZ1 and 3BZ2 are monomeric structures of the Photosystem II dimer) , , , , , .
| Protein Subunits (only with known function) | |
|---|---|
| Subunit | Function |
| D1 | Reaction center Protein, binds Chlorophyll P680, pheophytin, beta-carotene,quinone and manganese center |
| D2 | Reaction center Protein |
| CP43 | Binds manganese center |
| CP47 | |
| PsbO | Manganese Stabilizing Protein |
| Coenzymes/Cofactors | |
| Molecule | Function |
| Chlorophyll Chlorophyll Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light... |
Absorbs light |
| Beta-Carotene Beta-carotene β-Carotene is a strongly-coloured red-orange pigment abundant in plants and fruits. It is an organic compound and chemically is classified as a hydrocarbon and specifically as a terpenoid , reflecting its derivation from isoprene units... |
quench excess photoexcitation energy |
| Heme Heme A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are... b559 |
also Protoporphyrin IX Protoporphyrin IX Protoporphyrin IX, in the metabolism of porphyrin, is created by the enzyme protoporphyrinogen oxidase.-Heme b biosynthesis:In heme biosynthesis, the enzyme ferrochelatase converts it into heme b Protoporphyrin IX, in the metabolism of porphyrin, is created by the enzyme protoporphyrinogen... containing iron |
| Pheophytin Pheophytin Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of photosystem II in plants, and the photosynthetic reaction center found in purple bacteria... |
Primary electron acceptor |
| Plastoquinone Plastoquinone Plastoquinone is a quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. Plastoquinone is reduced , forming plastoquinol... |
Mobile intra-thylakoid membrane electron carrier |
| Manganese center | also known as the oxygen evolving center, or OEC |
Oxygen-Evolving Complex (OEC)
The oxygen-evolving complex is the site of water oxidation. It is a metallo-oxo cluster comprising four manganese ions (in oxidation states ranging from +3 to +5) and one divalent calcium ion. When it oxidizes water, producing dioxygen gas and protons, it sequentially delivers the four electrons from water to a tyrosine (D1-Y161) sidechain and then to P680 itself. The structure of the oxygen-evolving complex is still contentious. The structures obtained by X-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
are particularly controversial, since there is evidence that the manganese atoms are reduced by the high-intensity X-rays used, altering the observed OEC structure. However, crystallography in combination with a variety of other (less damaging) spectroscopic methods such as EXAFS and electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
have given a fairly clear idea of the structure of the cluster. One possibility is the cubane-like structure. In 2011 the OEC of PSII was resolved to a level of 1.9 angstroms revealing five oxygen atoms serving as oxo bridges linking the five metal atoms and four water molecules bound to the Mn4CaO5 cluster; more than 1,300 water molecules were found in each photosystem II monomer, some forming extensive hydrogen-bonding networks that may serve as channels for protons, water or oxygen molecules.
Water splitting
Photosynthetic water splitting (or oxygen evolutionOxygen evolution
Oxygen evolution is the process of generating molecular oxygen through chemical reaction. Mechanisms of oxygen evolution include the oxidation of water during oxygenic photosynthesis, electrolysis of water into oxygen and hydrogen, and electrocatalytic oxygen evolution from oxides and...
) is one of the most important reactions on the planet, since it is the source of nearly all the atmosphere's oxygen. Moreover, artificial photosynthetic water-splitting may contribute to the effective use of sunlight as an alternative energy-source.
The mechanism of water oxidation is still not fully elucidated, but we know many details about this process. The oxidation of water to molecular oxygen requires extraction of four electrons and four protons from two molecules of water. The experimental evidence that oxygen is released through cyclic reaction of oxygen evolving complex (OEC) within one PSII was provided by Pierre Joliot et al. They have shown that, if dark-adapted photosynthetic material (higher plants, algae, and cyanobacteria) is exposed to a series of single turnover flashes, oxygen evolution is detected with typical period-four damped oscillation with maxima on the third and the seventh flash and with minima on the first and the fifth flash (for review see ). Based on this experiment, Bessel Kok and co-workers introduced a cycle of five flash-induced transitions of the so-called S-states, describing the four redox states of OEC: When four oxidizing equivalents have been stored (at the S4-state), OEC returns to its basic and in the dark stable S0-state. Finally, the intermediate S-states were proposed by Jablonsky and Lazar as a regulatory mechanism and link between S-states and tyrosine Z.
See also
- PhotosynthesisPhotosynthesisPhotosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
- Oxygen evolutionOxygen evolutionOxygen evolution is the process of generating molecular oxygen through chemical reaction. Mechanisms of oxygen evolution include the oxidation of water during oxygenic photosynthesis, electrolysis of water into oxygen and hydrogen, and electrocatalytic oxygen evolution from oxides and...
- PhotosystemPhotosystemPhotosystems are functional and structural units of protein complexes involved in photosynthesis that together carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons...
- Reaction Centre
- P680P680P680, or Photosystem II primary donor, refers to any of the 2 special chlorophyll dimers , PD1 or PD2. These 2 special pairs form an excitonic dimer, which means that they behave in function as a single entity; i.e., they are excited as if they were a single molecule...
- Photosystem II light-harvesting protein
- Photosystem IPhotosystem IPhotosystem I is the second photosystem in the photosynthetic light reactions of algae, plants, and some bacteria. Photosystem I is so named because it was discovered before photosystem II. Aspects of PS I were discovered in the 1950s, but the significances of these discoveries was not yet known...

