Phthalocyanine
Encyclopedia
Phthalocyanine is an intensely blue-green coloured macrocyclic compound
that is widely used in dye
ing. Phthalocyanines form coordination complexes with most elements of the periodic table
. These complexes are also intensely colored and also are used as dyes or pigments.
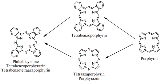
Phthalocyanines are structurally related to other macrocyclic pigments, especially the porphyrin
s. Both feature four pyrrole
-like subunits linked to form a 16-membered ring. The pyrrole-like rings within H2Pc are closely related to isoindole
. Both porphyrins and phthalocyanines function as planar tetradentate
dianionic ligand
s that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2-, the conjugate base of H2Pc.
Many derivatives of the parent phthalocyanine are known, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or where the hydrogen atoms of the ring are substituted by functional groups like halogens, hydroxy, amino, alkyl, aryl, thiol, alkoxy, nitro, etc. groups.
synthesized copper phthalocyanine, copper naphthalocyanine, and copper octamethylphthalocyanine in an attempted conversion of o-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize these blue complexes. In the same year, copper phthalocyanine was also discovered at Scottish Dyes, Ltd., Grangemouth, Scotland (later ICI
). ColorantHistory.org hosts an old documentary about the discovery of the pigment.
derivatives that contain nitrogen functional groups. Classical precursors are phthalonitrile
and diiminoisoindole. In the presence of urea
, the heating of phthalanhydride is a useful route to H2Pc. These reactions are more efficient in the presence of metal salts. Other precursors include , o-cyanobenzamide, , and phthalimide. Several of these starting materials are shown in the figure below (right side). Using such methods, approximately 57000 tons of various phthalocyanines were produced in 1985.
Halogenated
and sulfonated
derivatives of copper phthalocyanines are commercially important. Such compounds are prepared by treating CuPc with chlorine
, bromine
or oleum
.
functions. These dyes find extensive use in various areas of textile dye
ing (Direct dyes for cotton
), for spin dyeing and in the paper industry. Direct blue 86 is the sodium
salt of CPC-sulfonic acid
whereas direct blue 199 is the quaternary ammonium salt of the CPC-sulfonic acid. The quaternary ammonium salts of these sulfonic acids used as solvent dye
s because of their solubility in organic solvents, e.g. Solvent Blue 38 and Solvent Blue 48. The dye derived from cobalt phthalocyanine and an amine
is Phthalogen Dye IBN. 1,3-Diiminoisoindolene, the intermediate formed during phthalocyanine manufacture, used in combination with a copper salt affords the dye GK 161.
All major artists' pigment manufacturers produce variants of copper phthalocyanine, designated color index PB15 (blue) and color indexes PG7 and PG36 (green).
Phthalocyanine is also commonly used as a dye in the manufacture of high-speed CD-R
media. Most brands of CD-R use this dye except Taiyo Yuden
and Verbatim
DataLife (which use cyanine
and azo
dyes, respectively).
.
Phthalocyanine compounds have been investigated as donor materials in molecular electronics
, e.g. organic field-effect transistors.
macrocyles including porphyrins and porphyrazines
.
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
that is widely used in dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
ing. Phthalocyanines form coordination complexes with most elements of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
. These complexes are also intensely colored and also are used as dyes or pigments.
Properties
Unsubstituted phthalocyanine, abbreviated H2Pc, and many of its complexes have very low solubility in organic solvents. Benzene at 40 °C dissolves less than a milligram of H2Pc or CuPc per litre. H2Pc or CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are thermally very stable, do not melt but can be sublimed, CuPc sublimes at >500 °C under inert gases (nitrogen, CO2). Substituted phthalocyanine complexes often have much higher solubility. They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 to 700 nm, thus these materials are blue or green. Substitution can shift the absorption towards longer wavelengths, changing the color from pure blue to green to colorless (when the absorption is in the near infrared.Phthalocyanines are structurally related to other macrocyclic pigments, especially the porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
s. Both feature four pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
-like subunits linked to form a 16-membered ring. The pyrrole-like rings within H2Pc are closely related to isoindole
Isoindole
Isoindole in heterocyclic chemistry is a benzo fused pyrrole. The compound is an isomer of indole and its reduced form is an isoindoline.In solution its tautomer without full aromaticity over the whole ring system is predominant:...
. Both porphyrins and phthalocyanines function as planar tetradentate
Denticity
Denticity refers to the number of atoms in a single ligand that bind to a central atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate...
dianionic ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2-, the conjugate base of H2Pc.
Many derivatives of the parent phthalocyanine are known, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or where the hydrogen atoms of the ring are substituted by functional groups like halogens, hydroxy, amino, alkyl, aryl, thiol, alkoxy, nitro, etc. groups.
History
An unidentified blue compound, which is now known to be metal-free phthalocyanine, was first reported in 1907. In 1927, Swiss researchers accidentallySerendipity
Serendipity means a "happy accident" or "pleasant surprise"; specifically, the accident of finding something good or useful without looking for it. The word has been voted as one of the ten English words hardest to translate in June 2004 by a British translation company. However, due to its...
synthesized copper phthalocyanine, copper naphthalocyanine, and copper octamethylphthalocyanine in an attempted conversion of o-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize these blue complexes. In the same year, copper phthalocyanine was also discovered at Scottish Dyes, Ltd., Grangemouth, Scotland (later ICI
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
). ColorantHistory.org hosts an old documentary about the discovery of the pigment.
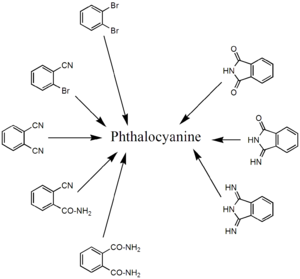
Synthesis
Phthalocyanine forms upon heating phthalic acidPhthalic acid
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H42. It is an isomer of isophthalic acid and terephthalic acid. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large...
derivatives that contain nitrogen functional groups. Classical precursors are phthalonitrile
Phthalonitrile
Phthalonitrile is an organic compound with the formula C6H42, which is an off-white crystal solid at room temperature. It is a benzene derivative, containing two adjacent nitrile groups. The compound is partially soluble in water, and is soluble in acetone, nitrobenzene, and benzonitrile...
and diiminoisoindole. In the presence of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, the heating of phthalanhydride is a useful route to H2Pc. These reactions are more efficient in the presence of metal salts. Other precursors include , o-cyanobenzamide, , and phthalimide. Several of these starting materials are shown in the figure below (right side). Using such methods, approximately 57000 tons of various phthalocyanines were produced in 1985.
 |
 |
Halogenated
Chlorination
Chlorination is the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water...
and sulfonated
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
derivatives of copper phthalocyanines are commercially important. Such compounds are prepared by treating CuPc with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
or oleum
Oleum
Oleum , or fuming sulfuric acid refers to a solution of various compositions of sulfur trioxide in sulfuric acid or sometimes more specifically to disulfuric acid ....
.
Applications
Approximately 25% of all artificial organic pigments are phthalocyanine derivatives. Copper phthalocyanine (CuPc) dyes are produced by introducing solubilizing groups, such as one or more sulfonic acidSulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
functions. These dyes find extensive use in various areas of textile dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
ing (Direct dyes for cotton
Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective capsule, around the seeds of cotton plants of the genus Gossypium. The fiber is almost pure cellulose. The botanical purpose of cotton fiber is to aid in seed dispersal....
), for spin dyeing and in the paper industry. Direct blue 86 is the sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
salt of CPC-sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
whereas direct blue 199 is the quaternary ammonium salt of the CPC-sulfonic acid. The quaternary ammonium salts of these sulfonic acids used as solvent dye
Solvent dye
A solvent dye is a dye soluble in organic solvents. It is usually used as a solution in an organic solvent. Solvent dyes are used to color organic solvents, hydrocarbon fuels, waxes, lubricants, plastics, and other hydrocarbon-based nonpolar materials. Fuel dyes are one use of solvent dyes. Their...
s because of their solubility in organic solvents, e.g. Solvent Blue 38 and Solvent Blue 48. The dye derived from cobalt phthalocyanine and an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
is Phthalogen Dye IBN. 1,3-Diiminoisoindolene, the intermediate formed during phthalocyanine manufacture, used in combination with a copper salt affords the dye GK 161.
All major artists' pigment manufacturers produce variants of copper phthalocyanine, designated color index PB15 (blue) and color indexes PG7 and PG36 (green).
Phthalocyanine is also commonly used as a dye in the manufacture of high-speed CD-R
CD-R
A CD-R is a variation of the Compact Disc invented by Philips and Sony. CD-R is a Write Once Read Many optical medium, though the whole disk does not have to be entirely written in the same session....
media. Most brands of CD-R use this dye except Taiyo Yuden
Taiyo Yuden
is a Japanese materials and electronics company, situated in Ueno, Taito, Tokyo, that helped pioneer recordable CD technology along with Sony and Philips in 1988. Founded 60 years ago, Taiyo Yuden currently operates factories in Japan, Singapore, Korea, China, the Philippines, Taiwan, Malaysia,...
and Verbatim
Verbatim Corporation
Verbatim Americas, LLC is a US company that markets storage media and flash memory products. It is a subsidiary of Mitsubishi Chemical Holdings Corporation of Japan and is based in Charlotte, North Carolina.-History:...
DataLife (which use cyanine
Cyanine
Cyanine is a non-systematic name of a synthetic dye family belonging to polymethine group. Cyanines have many uses as fluorescent dyes, particularly in biomedical imaging...
and azo
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
dyes, respectively).
Niche applications
Metal phthalocyanines have long been examined as catalysts for redox reactions. Areas of interest are the oxygen reduction reaction and the sweetening of gas streams by removal of hydrogen sulfideHydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
.
Phthalocyanine compounds have been investigated as donor materials in molecular electronics
Molecular electronics
Molecular electronics, sometimes called moletronics, involves the study and application of molecular building blocks for the fabrication of electronic components...
, e.g. organic field-effect transistors.
Toxicity and Hazards
There is evidence that exposure to phthalocyanines can cause serious birth defects in developing embryos.Related compounds
Phthalocyanines are closely related to other tetrapyrroleTetrapyrrole
Tetrapyrroles are compounds containing four pyrrole rings. With the exception of corrin, the four pyrrole rings are interconnected through one-carbon bridges, in either a linear or a cyclic fashion...
macrocyles including porphyrins and porphyrazines
Porphyrazine
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extenstion of his work on phthalocyanines, porphyrazines differ from porphyrins in that they contain -meso nitrogen atoms, rather than carbon...
.


