
Pitzer equations
Encyclopedia
Pitzer
equations are important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water. The parameters of the Pitzer equations are linear combinations of parameters, of a virial expansion
of the excess Gibbs free energy
, which characterise interactions amongst ions and solvent. The derivation is thermodynamically rigorous at a given level of expansion. The parameters may be determined by measuring osmotic coefficient
s. They can be used to calculate single-ion activity coefficient
s in solutions which for which Debye–Hückel theory
is inadequate. They are more rigorous than the equations of specific ion interaction theory
(SIT theory), but Pitzer parameters are more difficult to determine experimentally than SIT parameters.
where P is the pressure, V is the volume, T is the temperature and B, C, D ... are known as virial coefficients. The first term on the right-hand side is for an ideal gas
. The remaining terms quantify the departure from the ideal gas law
with changing pressure, P. It can be shown by statistical mechanics
that the second virial coefficient arises from the intermolecular forces between pairs of molecules, the third virial coefficient involves interactions between three molecules, etc. This theory was developed by McMillan and Mayer.
Solutions of uncharged molecules can be treated by a modification of the McMillan-Mayer theory. However when a solution contains electrolyte
s electrostatic interactions must also be taken into account. The Debye-Hückel theory was based on the assumption that each ion was surrounded by a spherical "cloud" made up of ions of the opposite charge. Expressions were derived for the variation of single-ion activity coefficient
s as a function of ionic strength
. This theory was very successful for dilute solutions of 1:1 electrolytes and, as discussed below, the Debye-Hückel expressions are still valid at sufficiently low concentrations. The values calculated with Debye-Hückel theory diverge more and more from observed values as the concentrations and/or ionic charges increases. Moreover, Debye-Hückel theory takes no account of the specific properties of ions such as size or shape.
Brønsted had independently proposed an empirical equation,
in which the activity coefficient depended not only on ionic strength, but also on the concentration, m, of the specific ion through the parameter β. This is the basis of SIT theory. It was further developed by Guggenheim. Scatchard extended the theory to allow the interaction coefficients to vary with ionic strength. Note that the second form of Brønsted's equation is an expression for the osmotic coefficient
. Measurement of osmotic coefficients provides one means for determining mean activity coefficients.
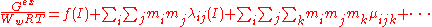
Ww is the mass of the water in kilograms, mi, mj ... are the molalities of the ions and I is the ionic strength. The first term, f(I) represents the Debye-Hückel limiting law. The quantities λij(I) represent the short-range interactions in the presence of solvent between solute particles i and j. This binary interaction parameter or second virial coefficient depends on ionic strength, on the particular species i and j and the temperature and pressure. The quantities μijk represent the interactions between three particles. Higher terms may also be included in the virial expansion.
Next, the free energy is expressed as the sum of chemical potential
s, or partial molal free energy,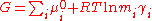
and an expression for the activity coefficient is obtained by differentiating the virial expansion with respect to a molality.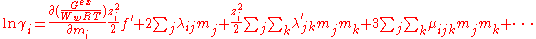
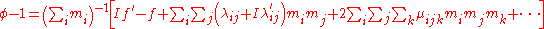
For a simple electrolyte MpXq, at a concentration m, made up of ions Mz+ and Xz−, the parameters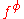 ,
, 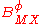 and
and 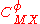
are defined as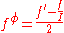
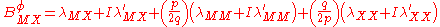
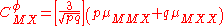
The term fφ is essentially the Debye-Hückel term. Terms involving and
and  are not included as interactions between three ions of the same charge are unlikely to occur except in very concentrated solutions.
are not included as interactions between three ions of the same charge are unlikely to occur except in very concentrated solutions.
The B parameter was found empirically to show an ionic strength dependence (in the absence of ion-pairing) which could be expressed as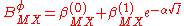
With these definitions, the expression for the osmotic coefficient becomes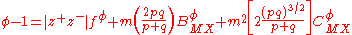
A similar expression is obtained for the mean activity coefficient.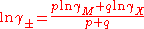
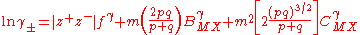
These equations were applied to an extensive range of experimental data at 25 °C with excellent agreement to about 6 mol kg−1 for various types of electrolyte. The treatment can be extended to mixed electrolytes
and to include association equilibria. Values for the parameters β(0), β(1) and C for inorganic and organic acids, bases and salts have been tabulated. Temperature and pressure variation is also discussed.
One area of application of Pitzer parameters is to describe the ionic strength variation of equilibrium constants measured as concentration quotients. Both SIT and Pitzer parameters have been used in this context, For example, both sets of parameters were calculated for some uranium
complexes and were found to account equally well for the ionic strength dependence of the stability constants.
Pitzer parameters and SIT theory have been extensively compared. There are more parameters in the Pitzer equations than in the SIT equations. Because of this the Pitzer equations provide for more precise modelling of mean activity coefficient data and equilibrium constants. However, the determination of the greater number of Pitzer parameters means that they are more difficult to determine.
related fields, were not summarized in their paper.
For some complex electrolytes, Ge et al. obtained the new set of Pitzer parameters using up-to-date measured or critically reviewed osmotic coefficient or activity coefficient data.
ln γ = ln γPDH + ln γSV
Ge et al. modified this model, and obtained the TCPC parameters for a larger number of single salts aqueous solutions. This model was also extended for a number of electrolytes dissolved in methanol,ethanol, 2-propanol, and so on. Temperature dependent parameters for a number of common single salts were also compiled, available at.
The performance of the TCPC model in correlation with the measured activity coefficient or osmotic coefficients is found to be comparable with Pitzer-like models.
Kenneth Pitzer
Kenneth Sanborn Pitzer was an American physical and theoretical chemist, educator, and university president....
equations are important for the understanding of the behaviour of ions dissolved in natural waters such as rivers, lakes and sea-water. The parameters of the Pitzer equations are linear combinations of parameters, of a virial expansion
Virial expansion
The classical virial expansion expresses the pressure of a many-particle system in equilibrium as a power series in the density.The virial expansion was introduced in 1901 by Heike Kamerlingh Onnesas a generalization of the ideal gas law...
of the excess Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
, which characterise interactions amongst ions and solvent. The derivation is thermodynamically rigorous at a given level of expansion. The parameters may be determined by measuring osmotic coefficient
Osmotic coefficient
An osmotic coefficient φ is a quantity which characterises the deviation of a solvent from ideal behaviour, referenced to Raoult's law. The osmotic coefficient on a molality basis is defined by:and on an amount fraction basis by:...
s. They can be used to calculate single-ion activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
s in solutions which for which Debye–Hückel theory
Debye–Hückel theory
The Debye–Hückel theory was proposed by Peter Debye and Erich Hückel as a theoretical explanation for departures from ideality in solutions of electrolytes. It was based on an extremely simplified model of the electrolyte solution but nevertheless gave accurate predictions of mean activity...
is inadequate. They are more rigorous than the equations of specific ion interaction theory
Specific ion interaction theory
Specific ion Interaction Theory is a theory used to estimate single-ion activity coefficients in electrolyte solutions at relatively high concentrations. It does so by taking into consideration interaction coefficients between the various ions present in solution...
(SIT theory), but Pitzer parameters are more difficult to determine experimentally than SIT parameters.
Historical development
A starting point for the development can be taken as the virial equation of state for a gas.- PV = RT + BP +CP2 + DP3 ...
where P is the pressure, V is the volume, T is the temperature and B, C, D ... are known as virial coefficients. The first term on the right-hand side is for an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
. The remaining terms quantify the departure from the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
with changing pressure, P. It can be shown by statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
that the second virial coefficient arises from the intermolecular forces between pairs of molecules, the third virial coefficient involves interactions between three molecules, etc. This theory was developed by McMillan and Mayer.
Solutions of uncharged molecules can be treated by a modification of the McMillan-Mayer theory. However when a solution contains electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s electrostatic interactions must also be taken into account. The Debye-Hückel theory was based on the assumption that each ion was surrounded by a spherical "cloud" made up of ions of the opposite charge. Expressions were derived for the variation of single-ion activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
s as a function of ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
. This theory was very successful for dilute solutions of 1:1 electrolytes and, as discussed below, the Debye-Hückel expressions are still valid at sufficiently low concentrations. The values calculated with Debye-Hückel theory diverge more and more from observed values as the concentrations and/or ionic charges increases. Moreover, Debye-Hückel theory takes no account of the specific properties of ions such as size or shape.
Brønsted had independently proposed an empirical equation,
- ln γ = −αm1/2 − 2βm
- 1 − φ = (α/3)m1/2 + βm
in which the activity coefficient depended not only on ionic strength, but also on the concentration, m, of the specific ion through the parameter β. This is the basis of SIT theory. It was further developed by Guggenheim. Scatchard extended the theory to allow the interaction coefficients to vary with ionic strength. Note that the second form of Brønsted's equation is an expression for the osmotic coefficient
Osmotic coefficient
An osmotic coefficient φ is a quantity which characterises the deviation of a solvent from ideal behaviour, referenced to Raoult's law. The osmotic coefficient on a molality basis is defined by:and on an amount fraction basis by:...
. Measurement of osmotic coefficients provides one means for determining mean activity coefficients.
The Pitzer parameters
The exposition begins with a virial expansion of the excess Gibbs free energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
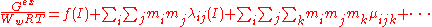
Ww is the mass of the water in kilograms, mi, mj ... are the molalities of the ions and I is the ionic strength. The first term, f(I) represents the Debye-Hückel limiting law. The quantities λij(I) represent the short-range interactions in the presence of solvent between solute particles i and j. This binary interaction parameter or second virial coefficient depends on ionic strength, on the particular species i and j and the temperature and pressure. The quantities μijk represent the interactions between three particles. Higher terms may also be included in the virial expansion.
Next, the free energy is expressed as the sum of chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
s, or partial molal free energy,
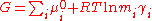
and an expression for the activity coefficient is obtained by differentiating the virial expansion with respect to a molality.
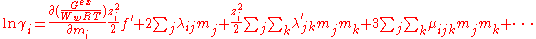
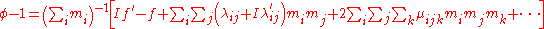
For a simple electrolyte MpXq, at a concentration m, made up of ions Mz+ and Xz−, the parameters
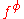 ,
, 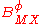 and
and 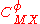
are defined as
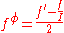
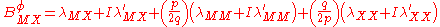
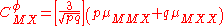
The term fφ is essentially the Debye-Hückel term. Terms involving
 and
and  are not included as interactions between three ions of the same charge are unlikely to occur except in very concentrated solutions.
are not included as interactions between three ions of the same charge are unlikely to occur except in very concentrated solutions.The B parameter was found empirically to show an ionic strength dependence (in the absence of ion-pairing) which could be expressed as
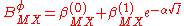
With these definitions, the expression for the osmotic coefficient becomes
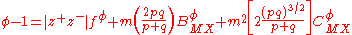
A similar expression is obtained for the mean activity coefficient.
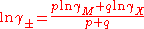
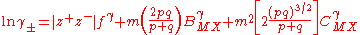
These equations were applied to an extensive range of experimental data at 25 °C with excellent agreement to about 6 mol kg−1 for various types of electrolyte. The treatment can be extended to mixed electrolytes
and to include association equilibria. Values for the parameters β(0), β(1) and C for inorganic and organic acids, bases and salts have been tabulated. Temperature and pressure variation is also discussed.
One area of application of Pitzer parameters is to describe the ionic strength variation of equilibrium constants measured as concentration quotients. Both SIT and Pitzer parameters have been used in this context, For example, both sets of parameters were calculated for some uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
complexes and were found to account equally well for the ionic strength dependence of the stability constants.
Pitzer parameters and SIT theory have been extensively compared. There are more parameters in the Pitzer equations than in the SIT equations. Because of this the Pitzer equations provide for more precise modelling of mean activity coefficient data and equilibrium constants. However, the determination of the greater number of Pitzer parameters means that they are more difficult to determine.
Compilation of Pitzer parameters
Besides the set of parameters obtained by Pitzer et al. in 1970s mentioned in the previous section. Kim and Frederick published the Pitzer parameters for 304 single salts in aqueous solutions at 298.15 K, extended the model to the concentration range up to the saturation point. Those parameters are widely used, however, many complex electrolytes including ones with organic anions or cations, which are very significant in somerelated fields, were not summarized in their paper.
For some complex electrolytes, Ge et al. obtained the new set of Pitzer parameters using up-to-date measured or critically reviewed osmotic coefficient or activity coefficient data.
A comparable TCPC model
Besides the well-known Pitzer-like equations, there is a simple and easy-to-use semi-empirical model, which is called the three-characteristic-parameter correlation(TCPC)model, which is first proposed by Lin et al. It is a combination of the Pitzer long-range interaction and short-range solvation effect.ln γ = ln γPDH + ln γSV
Ge et al. modified this model, and obtained the TCPC parameters for a larger number of single salts aqueous solutions. This model was also extended for a number of electrolytes dissolved in methanol,ethanol, 2-propanol, and so on. Temperature dependent parameters for a number of common single salts were also compiled, available at.
The performance of the TCPC model in correlation with the measured activity coefficient or osmotic coefficients is found to be comparable with Pitzer-like models.

