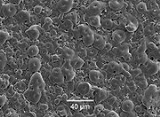
Plasma electrolytic oxidation
Encyclopedia
Plasma electrolytic oxidation (PEO), also known as microarc oxidation (MAO), is an electrochemical
surface treatment process for generating oxide
coatings on metals. It is similar to anodizing, but it employs higher potentials
, so that discharges
occur and the resulting plasma
modifies the structure of the oxide layer. This process can be used to grow thick (tens or hundreds of micrometers), largely crystalline, oxide coatings on metals such as aluminium
, magnesium
and titanium
. Because they can present high hardness and a continuous barrier, these coatings can offer protection against wear
, corrosion
or heat as well as electrical insulation.

 The coating is a chemical conversion of the substrate metal into its oxide
The coating is a chemical conversion of the substrate metal into its oxide
, and grows both inwards and outwards from the original metal surface. Because it is a conversion coating, rather than a deposited coating (such as a coating formed by plasma spraying), it has excellent adhesion
to the substrate metal. A wide range of substrate alloys can be coated, including all wrought aluminium alloys and most cast alloys, although high levels of silicon can reduce coating quality.
oxide layer which provides moderate protection against corrosion. The layer is strongly adherent
to the metal surface, and it will regrow quickly if scratched off. In conventional anodizing, this layer of oxide is grown on the surface of the metal by the application of electrical potential
, while the part is immersed in an acidic electrolyte
.
In plasma electrolytic oxidation, higher potentials
are applied. For example, in the plasma electrolytic oxidation of aluminium, at least 200 V must be applied. This locally exceeds the dielectric breakdown potential
of the growing oxide film, and discharges
occur. These discharges result in localised plasma reactions, with conditions of high temperature and pressure which modify the growing oxide. Processes include melting, melt-flow, re-solidification, sintering
and densification of the growing oxide. One of the most significant effects, is that the oxide is partially converted from amorphous alumina into crystalline forms such as corundum
(α-Al2O3) which is much harder. As a result, mechanical properties such as wear
resistance and toughness
are enhanced.
which usually consists of a dilute alkaline solution
such as KOH. It is electrically connected, so as to become one of the electrodes in the electrochemical cell
, with the other, being a stainless steel
counter-electrode, often the wall of the bath itself.
Potentials of over 200 V are applied between these two electrodes. These may be continuous or pulsed direct current
(DC) (in which case the part is simply an anode
in DC operation), or alternating pulses (alternating current
or "pulsed bi-polar" operation) where the stainless steel counter electrode might just be earthed
.
Even on aluminium, the coating properties can vary strongly according to the exact alloy
composition. For instance, the hardest coatings can be achieved on 2XXX series aluminium alloy
s, where the highest proportion of crystalline phase corundum
(α-Al2O3) is formed, resulting in hardnesses of ~2000 HV, whereas coatings on the 5XXX series have less of this important constituent and are hence softer. Extensive work is being pursued by Prof. T. W. Clyne at the University of Cambridge
to investigate the fundamental electrical and plasma physical processes involved in this process, having previously elucidated some of the micromechanical (& pore architectural), mechanical and thermal characteristics of PEO coatings.
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
surface treatment process for generating oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
coatings on metals. It is similar to anodizing, but it employs higher potentials
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
, so that discharges
Electrostatic discharge
Electrostatic discharge is a serious issue in solid state electronics, such as integrated circuits. Integrated circuits are made from semiconductor materials such as silicon and insulating materials such as silicon dioxide...
occur and the resulting plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
modifies the structure of the oxide layer. This process can be used to grow thick (tens or hundreds of micrometers), largely crystalline, oxide coatings on metals such as aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
and titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
. Because they can present high hardness and a continuous barrier, these coatings can offer protection against wear
Wear
In materials science, wear is erosion or sideways displacement of material from its "derivative" and original position on a solid surface performed by the action of another surface....
, corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
or heat as well as electrical insulation.


Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
, and grows both inwards and outwards from the original metal surface. Because it is a conversion coating, rather than a deposited coating (such as a coating formed by plasma spraying), it has excellent adhesion
Adhesion
Adhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
to the substrate metal. A wide range of substrate alloys can be coated, including all wrought aluminium alloys and most cast alloys, although high levels of silicon can reduce coating quality.
Process
Metals such as aluminium naturally form a passivatingPassivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
oxide layer which provides moderate protection against corrosion. The layer is strongly adherent
Adhesion
Adhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
to the metal surface, and it will regrow quickly if scratched off. In conventional anodizing, this layer of oxide is grown on the surface of the metal by the application of electrical potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
, while the part is immersed in an acidic electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
.
In plasma electrolytic oxidation, higher potentials
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
are applied. For example, in the plasma electrolytic oxidation of aluminium, at least 200 V must be applied. This locally exceeds the dielectric breakdown potential
Dielectric strength
In physics, the term dielectric strength has the following meanings:*Of an insulating material, the maximum electric field strength that it can withstand intrinsically without breaking down, i.e., without experiencing failure of its insulating properties....
of the growing oxide film, and discharges
Electrostatic discharge
Electrostatic discharge is a serious issue in solid state electronics, such as integrated circuits. Integrated circuits are made from semiconductor materials such as silicon and insulating materials such as silicon dioxide...
occur. These discharges result in localised plasma reactions, with conditions of high temperature and pressure which modify the growing oxide. Processes include melting, melt-flow, re-solidification, sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
and densification of the growing oxide. One of the most significant effects, is that the oxide is partially converted from amorphous alumina into crystalline forms such as corundum
Corundum
Corundum is a crystalline form of aluminium oxide with traces of iron, titanium and chromium. It is a rock-forming mineral. It is one of the naturally clear transparent materials, but can have different colors when impurities are present. Transparent specimens are used as gems, called ruby if red...
(α-Al2O3) which is much harder. As a result, mechanical properties such as wear
Wear
In materials science, wear is erosion or sideways displacement of material from its "derivative" and original position on a solid surface performed by the action of another surface....
resistance and toughness
Toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing; Material toughness is defined as the amount of energy per volume that a material can absorb before rupturing...
are enhanced.
Equipment used
The part to be coated is immersed in a bath of electrolyteElectrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
which usually consists of a dilute alkaline solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
such as KOH. It is electrically connected, so as to become one of the electrodes in the electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
, with the other, being a stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
counter-electrode, often the wall of the bath itself.
Potentials of over 200 V are applied between these two electrodes. These may be continuous or pulsed direct current
Direct current
Direct current is the unidirectional flow of electric charge. Direct current is produced by such sources as batteries, thermocouples, solar cells, and commutator-type electric machines of the dynamo type. Direct current may flow in a conductor such as a wire, but can also flow through...
(DC) (in which case the part is simply an anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
in DC operation), or alternating pulses (alternating current
Alternating current
In alternating current the movement of electric charge periodically reverses direction. In direct current , the flow of electric charge is only in one direction....
or "pulsed bi-polar" operation) where the stainless steel counter electrode might just be earthed
Ground (electricity)
In electrical engineering, ground or earth may be the reference point in an electrical circuit from which other voltages are measured, or a common return path for electric current, or a direct physical connection to the Earth....
.
Coating properties
Plasma electrolytic oxide coatings are generally recognized for high hardness, wear resistance, and corrosion resistance. However, the coating properties are highly dependent on the substrate used, as well as on the composition of the electrolyte and the electrical regime used (see 'Equipment used' section, above).Even on aluminium, the coating properties can vary strongly according to the exact alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
composition. For instance, the hardest coatings can be achieved on 2XXX series aluminium alloy
Aluminium alloy
Aluminium alloys are alloys in which aluminium is the predominant metal. The typical alloying elements are copper, magnesium, manganese, silicon and zinc. There are two principal classifications, namely casting alloys and wrought alloys, both of which are further subdivided into the categories...
s, where the highest proportion of crystalline phase corundum
Corundum
Corundum is a crystalline form of aluminium oxide with traces of iron, titanium and chromium. It is a rock-forming mineral. It is one of the naturally clear transparent materials, but can have different colors when impurities are present. Transparent specimens are used as gems, called ruby if red...
(α-Al2O3) is formed, resulting in hardnesses of ~2000 HV, whereas coatings on the 5XXX series have less of this important constituent and are hence softer. Extensive work is being pursued by Prof. T. W. Clyne at the University of Cambridge
University of Cambridge
The University of Cambridge is a public research university located in Cambridge, United Kingdom. It is the second-oldest university in both the United Kingdom and the English-speaking world , and the seventh-oldest globally...
to investigate the fundamental electrical and plasma physical processes involved in this process, having previously elucidated some of the micromechanical (& pore architectural), mechanical and thermal characteristics of PEO coatings.

