Retrosynthetic analysis
Encyclopedia
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses
. This is achieved by transforming a target molecule into simpler precursor structures without assumptions regarding starting materials. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. E.J. Corey formalized this concept in his book The Logic of Chemical Synthesis.
The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightfoward fashion. A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required.
Retron: A minimal molecular substructure that enables certain transformations.
Retrosynthetic tree: A directed acyclic graph
of several (or all) possible retrosyntheses of a single target.
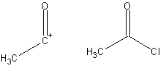
Synthon
: An idealized molecular fragment. A synthon and the corresponding commercially available synthetic equivalent are shown below:
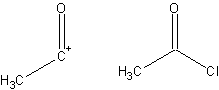
Target: The desired final compound.
Transform: The exact reverse of a synthetic reaction; the formation of starting materials from a single product.
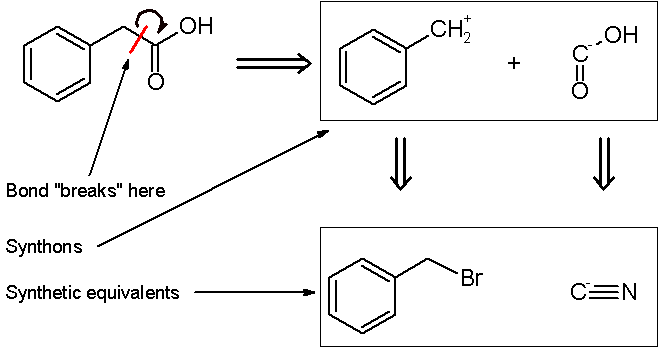 In planning the synthesis of phenylacetic acid
In planning the synthesis of phenylacetic acid
, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the -COOH synthon, while benzyl bromide
is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
In fact, phenylacetic acid has been synthesized from benzyl cyanide
, itself prepared by the analogous reaction of benzyl chloride
with sodium cyanide
.
Functional Group
Manipulation of functional groups can lead to significant reductions in molecular complexity.
Stereochemical
Numerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangement
and Mitsunobu reaction
) can remove or transfer the desired chirality thus simplifying the target.
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. This is achieved by transforming a target molecule into simpler precursor structures without assumptions regarding starting materials. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. E.J. Corey formalized this concept in his book The Logic of Chemical Synthesis.
The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightfoward fashion. A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required.
Definitions
Disconnection: A retrosynthetic step involving the breaking of a bond to form two (or more) synthons.Retron: A minimal molecular substructure that enables certain transformations.
Retrosynthetic tree: A directed acyclic graph
Directed acyclic graph
In mathematics and computer science, a directed acyclic graph , is a directed graph with no directed cycles. That is, it is formed by a collection of vertices and directed edges, each edge connecting one vertex to another, such that there is no way to start at some vertex v and follow a sequence of...
of several (or all) possible retrosyntheses of a single target.
Synthon
Synthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
: An idealized molecular fragment. A synthon and the corresponding commercially available synthetic equivalent are shown below:
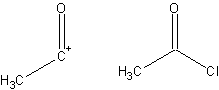
Target: The desired final compound.
Transform: The exact reverse of a synthetic reaction; the formation of starting materials from a single product.
Example
An example will allow the concept of retrosynthetic analysis to be easily understood.
Phenylacetic acid
Phenylacetic acid is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a disagreeable odor...
, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the -COOH synthon, while benzyl bromide
Benzyl bromide
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese oxide as a heterogeneous catalyst...
is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
- PhCH2Br + NaCN → PhCH2CN + NaBr
- PhCH2CN + 2 H2O → PhCH2COOH + NH3
In fact, phenylacetic acid has been synthesized from benzyl cyanide
Benzyl cyanide
Benzyl cyanide is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is a precursor to several derivatives.-Synthesis, reactions, and applications:...
, itself prepared by the analogous reaction of benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
with sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
.
Functional GroupFunctional groupIn organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
Strategies
Manipulation of functional groups can lead to significant reductions in molecular complexity.StereochemicalStereochemistryStereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
Strategies
Numerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangementClaisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
and Mitsunobu reaction
Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate or diisopropyl azodicarboxylate . The alcohol undergoes an inversion of stereochemistry...
) can remove or transfer the desired chirality thus simplifying the target.
Structure-Goal Strategies
Directing a synthesis toward a desirable intermediate can greatly narrow the focus of an analysis. This allows bidirectional search techniques.Transform-based Strategies
The application of transformations to retrosynthetic analysis can lead to powerful reductions in molecular complexity. Unfortunately, powerful transform-based retrons are rarely present in complex molecules, and additional synthetic steps are often needed to establish their presence.Topological Strategies
The identification one or more key bond disconnections may lead to the identification of key substructures or difficult to identify rearrangement transformations.- Disconnections that preserve ring structures are encouraged.
- Disconnections that create rings larger than 7 members are discouraged.
External links
- Centre for Molecular and Biomolecular Informatics
- Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008 for more info on ARChem see the SimBioSysSimbiosysSimBioSys is a Toronto based chemistry software company focusing on structure based drug discovery and retrosynthetic analysis tools...
pages.

