Rubredoxin
Encyclopedia
Rubredoxins are a class of low-molecular-weight iron
-containing proteins found in sulfur-metabolizing bacteria
and archaea
. Sometimes rubredoxins are classified as iron-sulfur protein
s; however, in contrast to iron-sulfur proteins, rubredoxins do not contain inorganic sulfide.
Like cytochrome
s, ferredoxin
s and Rieske protein
s, rubredoxins participate in electron transfer
in biological systems.
residues forming an almost regular tetrahedron. This is sometimes denoted as a [1Fe-0S] or an Fe1S0 system, in analogy to the nomenclature for iron-sulfur proteins.
Rubredoxins perform one-electron transfer processes. The central iron atom changes between the +2 and +3 oxidation state
s. In both oxidation states, the metal remains high spin, which helps to minimize structural changes. The reduction potential
of a rubredoxin is typically in the range +50 mV to -50 mV.
This iron-sulphur protein is an electron carrier, and it is easy to distinguish its metallic centre changes: the oxidized state is reddish (due to a ligand metal charge transfer), while the reduced state is colourless (because the electron transition has an energy of the infrared level, which is imperceptible for the human eye).
alkane 1-monooxygenase (alkane,reduced-rubredoxin:oxygen 1-oxidoreductase)
superoxide reductase (rubredoxin:superoxide oxidoreductase)
rubredoxin—NAD+ reductase (rubredoxin:NAD+ oxidoreductase)
rubredoxin—NAD(P)+ reductase (rubredoxin:NAD(P)+ oxidoreductase)
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-containing proteins found in sulfur-metabolizing bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
. Sometimes rubredoxins are classified as iron-sulfur protein
Iron-sulfur protein
Iron-sulfur proteins are proteins characterized by the presence of iron-sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states...
s; however, in contrast to iron-sulfur proteins, rubredoxins do not contain inorganic sulfide.
Like cytochrome
Cytochrome
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
s, ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
s and Rieske protein
Rieske protein
Rieske proteins are iron-sulfur protein components of cytochrome bc1 complexes and cytochrome b6f complexes which were first discovered and isolated by John S. Rieske and co-workers in 1964. It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues...
s, rubredoxins participate in electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
in biological systems.
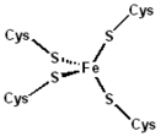
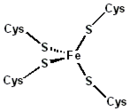
Structure
The 3-D structures of a number of rubredoxins have been solved. The fold belongs to the α+β class, with 2 α-helices and 2-3 β-strands. Rubredoxin active site contains an iron ion which is coordinated by the sulfurs of four conserved cysteineCysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues forming an almost regular tetrahedron. This is sometimes denoted as a [1Fe-0S] or an Fe1S0 system, in analogy to the nomenclature for iron-sulfur proteins.
Rubredoxins perform one-electron transfer processes. The central iron atom changes between the +2 and +3 oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s. In both oxidation states, the metal remains high spin, which helps to minimize structural changes. The reduction potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
of a rubredoxin is typically in the range +50 mV to -50 mV.
This iron-sulphur protein is an electron carrier, and it is easy to distinguish its metallic centre changes: the oxidized state is reddish (due to a ligand metal charge transfer), while the reduced state is colourless (because the electron transition has an energy of the infrared level, which is imperceptible for the human eye).

Rubredoxin in some biochemical reactions
camphor 1,2-monooxygenase [(+)-camphor,reduced-rubredoxin:oxygen oxidoreductase (1,2-lactonizing)]- (+)-bornane-2,5-dione + reduced rubredoxin + O2 = 5-oxo-1,2-campholide + oxidized rubredoxin + H2O
alkane 1-monooxygenase (alkane,reduced-rubredoxin:oxygen 1-oxidoreductase)
- octane + reduced rubredoxin + O2 = 1-octanol + oxidized rubredoxin + H2O
superoxide reductase (rubredoxin:superoxide oxidoreductase)
- reduced rubredoxin + superoxide + 2 H+ = rubredoxin + H2O2
rubredoxin—NAD+ reductase (rubredoxin:NAD+ oxidoreductase)
- reduced rubredoxin + NAD+ = oxidized rubredoxin + NADH + H+
rubredoxin—NAD(P)+ reductase (rubredoxin:NAD(P)+ oxidoreductase)
- reduced rubredoxin + NAD(P)+ = oxidized rubredoxin + NAD(P)H + H+
See also
- Bioinorganic chemistryBioinorganic chemistryBioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well artificially introduced metals, including those that are non-essential, in medicine and toxicology...
- Iron-sulfur proteinIron-sulfur proteinIron-sulfur proteins are proteins characterized by the presence of iron-sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states...
- FerredoxinFerredoxinFerredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
- CytochromeCytochromeCytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.They are found either as monomeric proteins or as subunits of bigger enzymatic complexes that catalyze redox reactions....
- Rieske proteinRieske proteinRieske proteins are iron-sulfur protein components of cytochrome bc1 complexes and cytochrome b6f complexes which were first discovered and isolated by John S. Rieske and co-workers in 1964. It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues...

