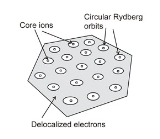
Rydberg matter
Encyclopedia
Rydberg matter is a phase of matter
formed by Rydberg atom
s; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium
, potassium
, hydrogen
and nitrogen
; studies have been conducted on theoretical possibilities like sodium
, beryllium
, magnesium
and calcium
. It has been suggested to be a material that diffuse interstellar band
s may arise from; circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived with lifetimes of up to several hours and are the most common. This hypothesis, however, is disputed and is not generally accepted by the astronomical community.

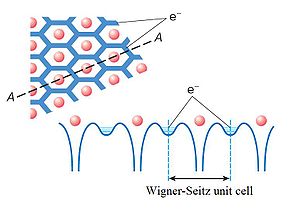 Rydberg matter consists of usually hexagonal planar clusters
Rydberg matter consists of usually hexagonal planar clusters
; these cannot be very big because of the retardation effect caused by the finite velocity of the speed of light. Hence, they are not gases or plasmas; nor are they solids or liquids; they are most similar to dusty plasma
s with small clusters in a gas. Though Rydberg matter can be studied in the laboratory by laser probing, the largest cluster reported consists of only 91 atoms, but it has been shown to be behind extended clouds in space and the upper atmosphere of planets. Bonding in Rydberg matter is caused by delocalisation of the high-energy electrons to form an overall lower energy state. The way in which the electrons delocalise is to form standing waves on loops surrounding nuclei, creating quantised angular momentum and the defining characteristics of Rydberg matter. It is a generalised metal by way of the quantum numbers influencing loop size but restricted by the bonding requirement for strong electron correlation; it shows exchange-correlation properties similar to covalent bonding. Electronic excitation and vibrational motion of these bonds can be studied by Raman spectroscopy
.
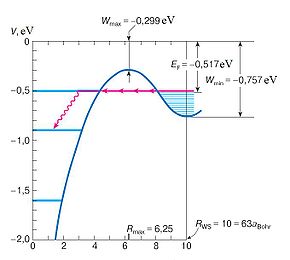 Due to reasons still debated by the physics community because of the lack of methods to observe clusters, Rydberg matter is highly stable against disintegration by emission of radiation; the characteristic lifetime of a cluster at n = 100 is 17 seconds. Reasons given include the lack of overlap between excited and ground states, the forbidding of transitions between them and exchange-correlation effects hindering emission through necessitating tunnelling that causes a long delay in excitation decay. Excitation plays a role in determining lifetimes, with a higher excitation giving a longer lifetime; n = 80 gives a lifetime comparable to the age of the Universe.
Due to reasons still debated by the physics community because of the lack of methods to observe clusters, Rydberg matter is highly stable against disintegration by emission of radiation; the characteristic lifetime of a cluster at n = 100 is 17 seconds. Reasons given include the lack of overlap between excited and ground states, the forbidding of transitions between them and exchange-correlation effects hindering emission through necessitating tunnelling that causes a long delay in excitation decay. Excitation plays a role in determining lifetimes, with a higher excitation giving a longer lifetime; n = 80 gives a lifetime comparable to the age of the Universe.
In ordinary metals, interatomic distances are nearly constant through a wide range of temperatures and pressures; this is not the case with Rydberg matter, whose distances and thus properties vary greatly with excitations. A key variable in determining these properties is the principal quantum number n that can be any integer greater than 1; the highest values reported for it are around 100. Bond distance d in Rydberg matter is given by
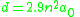
where a0 is the Bohr radius
. The approximate factor 2.9 was first experimentally determined, then measured with rotational spectroscopy in different clusters. Examples of d calculated this way, along with selected values of the density D, are given in the table to the right.
s that can be condensed to form Bose-Einstein condensates, Rydberg matter can be condensed, but not in the same way as bosons. The reason for this is that Rydberg matter behaves similar to a gas, meaning that it cannot be condensed without removing the condensation energy; ionisation occurs if this is not done. All solutions to this problem so far involve using an adjacent surface in some way, the best being evaporating the atoms of which the Rydberg matter is to be formed from and leaving the condensation energy on the surface. Using caesium
atoms, graphite-covered surfaces and thermionic converter
s as containment, the work function
of the surface has been measured to be 0.5eV, indicating that the cluster is between the ninth and fourteenth excitation levels.
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
formed by Rydberg atom
Rydberg atom
thumb|right|300px|Figure 1: Energy levels in atomic [[lithium]] showing the Rydberg series of the lowest 3 values of [[Angular momentum#Angular momentum in quantum mechanics|orbital angular momentum]] converging on the first ionization energy....
s; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
; studies have been conducted on theoretical possibilities like sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
, magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
and calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
. It has been suggested to be a material that diffuse interstellar band
Diffuse interstellar band
Diffuse interstellar bands are absorption features seen in the spectra of astronomical objects in our galaxy. They are caused by the absorption of light by the interstellar medium...
s may arise from; circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived with lifetimes of up to several hours and are the most common. This hypothesis, however, is disputed and is not generally accepted by the astronomical community.
Physical


Cluster (physics)
In physics, the term clusters denotes small, multiatom particles. As a rule of thumb, any particle of somewhere between 3 and 3x107 atoms is considered a cluster. Two-atom particles are sometimes considered clusters as well....
; these cannot be very big because of the retardation effect caused by the finite velocity of the speed of light. Hence, they are not gases or plasmas; nor are they solids or liquids; they are most similar to dusty plasma
Dusty plasma
A dusty plasma is a plasma containing nanometer or micrometer-sized particles suspended in it. Dust particles may be charged and the plasma and particles behave as a plasma, following electromagnetic laws for particles up to about 10 nm...
s with small clusters in a gas. Though Rydberg matter can be studied in the laboratory by laser probing, the largest cluster reported consists of only 91 atoms, but it has been shown to be behind extended clouds in space and the upper atmosphere of planets. Bonding in Rydberg matter is caused by delocalisation of the high-energy electrons to form an overall lower energy state. The way in which the electrons delocalise is to form standing waves on loops surrounding nuclei, creating quantised angular momentum and the defining characteristics of Rydberg matter. It is a generalised metal by way of the quantum numbers influencing loop size but restricted by the bonding requirement for strong electron correlation; it shows exchange-correlation properties similar to covalent bonding. Electronic excitation and vibrational motion of these bonds can be studied by Raman spectroscopy
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
.
Lifetime

Excitations
| n | d (nm) | D (cm−3) |
|---|---|---|
| 1 | 0.153 | 2.8×1023 |
| 4 | 2.45 | |
| 5 | 3.84 | |
| 6 | 5.52 | |
| 10 | 15.3 | 2.8×1017 |
| 40 | 245 | |
| 80 | 983 | |
| 100 | 1534 | 2.8×1011 |
In ordinary metals, interatomic distances are nearly constant through a wide range of temperatures and pressures; this is not the case with Rydberg matter, whose distances and thus properties vary greatly with excitations. A key variable in determining these properties is the principal quantum number n that can be any integer greater than 1; the highest values reported for it are around 100. Bond distance d in Rydberg matter is given by
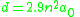
where a0 is the Bohr radius
Bohr radius
The Bohr radius is a physical constant, approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom...
. The approximate factor 2.9 was first experimentally determined, then measured with rotational spectroscopy in different clusters. Examples of d calculated this way, along with selected values of the density D, are given in the table to the right.
Condensation
Like bosonBoson
In particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose....
s that can be condensed to form Bose-Einstein condensates, Rydberg matter can be condensed, but not in the same way as bosons. The reason for this is that Rydberg matter behaves similar to a gas, meaning that it cannot be condensed without removing the condensation energy; ionisation occurs if this is not done. All solutions to this problem so far involve using an adjacent surface in some way, the best being evaporating the atoms of which the Rydberg matter is to be formed from and leaving the condensation energy on the surface. Using caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
atoms, graphite-covered surfaces and thermionic converter
Thermionic converter
A thermionic converter consists of a hot electrode which thermionically emits electrons over a potential energy barrier to a cooler electrode, producing a useful electric power output...
s as containment, the work function
Work function
In solid-state physics, the work function is the minimum energy needed to remove an electron from a solid to a point immediately outside the solid surface...
of the surface has been measured to be 0.5eV, indicating that the cluster is between the ninth and fourteenth excitation levels.

