Silylene
Encyclopedia
Silylenes are chemical compound
s containing a divalent
silicon
atom
without any electrical charge. Both dicoordinate and tricoordinate silylenes are reported in the literature. They are considered to be heavier analogues of carbene
. In earlier times, they were called silene
, but this is a mistake, as that term means compound
s of π-bond
ed silicon
: Si=C. The generic term analogous to carbene
is "silicene."
Silylenes have been proposed as reactive intermediate
s and are so unstable that they usually cannot be isolated. While carbene
s are observed in either the triplet
or singlet state depending on the nature of the substituents, silylenes typically have a singlet
ground state
because the energy gap between the 3s
and 3p
orbital
s of the silicon
atom is very large and so the singlet-triplet gaps are enormous.
The first stable silylene was synthesized and isolated as a diamino silylene, N,N’-di-tert-butyl-1,3-diaza-2-silacyclopent-4-en-2-ylidene, in 1994 by M. Denk
et al.
s, by silicon
atom reactions (insertion
, addition
or abstraction), by pyrolysis
of silane
s, or by reduction
of 1,1-dihalosilane.
Simple silylenes are very reactive species and easily dimerize to give disilene
s, so their lifetime is very short. Bulky substituent
s are used to prevent this dimerization and stabilize silylenes effectively for long-term survival in dilute solution and even in crystals.
The reactivities of silylenes are similar to those of carbene
s, and insertion
to polar bonds, addition
to multiple bond
s, and dimerization occur. But, in contrast to carbene
s, insertion
to C-H bonds and C-C bonds generally does not happen and complexation to Lewis base takes place.
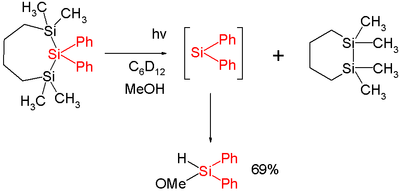
In one study diphenylsilylene is generated by flash photolysis
of a trisilane :
In this reaction diphenylsilylene is simply extruded with formation of the disilane. The silylene can be observed with UV spectroscopy at 520 nm and is short-lived with a chemical half-life of 2microsecond
s. Added methanol
acts as a chemical trap
with a second order rate constant of 13x109 mol−1s−1 which is close to diffusion control.
s, silylenes can be stabilized. The first stable silylenes were discovered by Robert West
at the University of Wisconsin–Madison
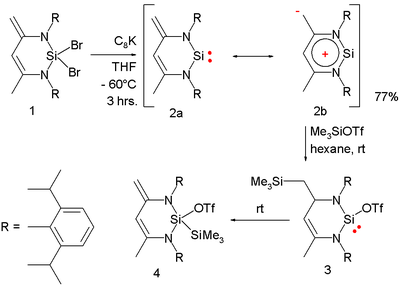
. In one study a stable silylene is synthesized by debromination the dibromosilyl compound 1 by potassium graphite:
The α-nitrogen atoms in 2 stabilize the electrophilic silicon atom by п-donation. In one contributing resonance
structure 2b a negative charge is centered in an exocyclic methylene group and a positive charge delocalized over the silicon ring system. X-ray diffraction of the compound shows equal N-Si bonds (173 pm) and an unusually short exocylic bond with bond length
of 143.6 pm. The compound reacts with trimethylsilyltriflate to the 1,4-adduct 3 (kinetic product) which slowly isomerizes to the 1,1-adduct 4 as the thermodynamically controlled
product.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s containing a divalent
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
without any electrical charge. Both dicoordinate and tricoordinate silylenes are reported in the literature. They are considered to be heavier analogues of carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
. In earlier times, they were called silene
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
, but this is a mistake, as that term means compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s of π-bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
ed silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
: Si=C. The generic term analogous to carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
is "silicene."
Silylenes have been proposed as reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s and are so unstable that they usually cannot be isolated. While carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s are observed in either the triplet
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
or singlet state depending on the nature of the substituents, silylenes typically have a singlet
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
because the energy gap between the 3s
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
and 3p
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
orbital
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
s of the silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
atom is very large and so the singlet-triplet gaps are enormous.
History
The first experimental chemical observation of silylene as an intermediate was demonstrated by P.S. Skell and E.J. Goldstein in 1964.The first stable silylene was synthesized and isolated as a diamino silylene, N,N’-di-tert-butyl-1,3-diaza-2-silacyclopent-4-en-2-ylidene, in 1994 by M. Denk
Michael K. Denk
Michael K. Denk is a Professor of chemistry at the University of Guelph, Ontario.Michael Denk obtained his M.Sc. at the Ludwig Maximilian University in Munich, Germany . He got his Ph.D. at the Technical University of Munich , advised by W. Herrmann, with a dissertation on cyclic metalloamides...
et al.
Synthesis and properties
Silylenes are generally synthesized by thermolysis or photolysis of polysilaneSilanes
Silanes are chemical compounds of silicon and hydrogen, which are analogues of alkane hydrocarbons. Silanes consist of a chain of silicon atoms covalently bonded to each other and to hydrogen atoms. The general formula of a silane is SinH2n+2...
s, by silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
atom reactions (insertion
Insertion
Insertion may refer to:*Insertion , the point of a tendon or ligament onto the skeleton or other part of the body*Insertion , the addition of DNA into a genetic sequence*Insertion loss, in electronics...
, addition
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
or abstraction), by pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
of silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
s, or by reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of 1,1-dihalosilane.
Simple silylenes are very reactive species and easily dimerize to give disilene
Disilene
Disilenes are compounds containing a silicon–silicon double bond and are considered to be heavier analogues of alkenes. They are sometimes also called disilaalkenes.-History:...
s, so their lifetime is very short. Bulky substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s are used to prevent this dimerization and stabilize silylenes effectively for long-term survival in dilute solution and even in crystals.
The reactivities of silylenes are similar to those of carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s, and insertion
Insertion
Insertion may refer to:*Insertion , the point of a tendon or ligament onto the skeleton or other part of the body*Insertion , the addition of DNA into a genetic sequence*Insertion loss, in electronics...
to polar bonds, addition
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
to multiple bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s, and dimerization occur. But, in contrast to carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s, insertion
Insertion
Insertion may refer to:*Insertion , the point of a tendon or ligament onto the skeleton or other part of the body*Insertion , the addition of DNA into a genetic sequence*Insertion loss, in electronics...
to C-H bonds and C-C bonds generally does not happen and complexation to Lewis base takes place.
In one study diphenylsilylene is generated by flash photolysis
Flash photolysis
Flash photolysis is a pump-probe laboratory technique, in which a sample is firstly excited by a strong pulse of light from a laser of nanosecond, picosecond, or femtosecond pulse width or by a short-pulse light source such as a flash lamp...
of a trisilane :
In this reaction diphenylsilylene is simply extruded with formation of the disilane. The silylene can be observed with UV spectroscopy at 520 nm and is short-lived with a chemical half-life of 2microsecond
Microsecond
A microsecond is an SI unit of time equal to one millionth of a second. Its symbol is µs.A microsecond is equal to 1000 nanoseconds or 1/1000 millisecond...
s. Added methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
acts as a chemical trap
Chemical trap
In chemistry, a chemical trap is a chemical compound that is used to detect a certain molecule when* The concentration of this molecule is very small and below detection limit...
with a second order rate constant of 13x109 mol−1s−1 which is close to diffusion control.
Persistent silylenes
Just as with persistent carbenePersistent carbene
A persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
s, silylenes can be stabilized. The first stable silylenes were discovered by Robert West
Robert West
Robert C. West is E. G. Rochow Professor of Chemistry Emeritus at the University of Wisconsin–Madison; Director of the Organosilicon Research Center, University of Wisconsin–Madison 1999–present; President, Silatronix, Inc...
at the University of Wisconsin–Madison
University of Wisconsin–Madison
The University of Wisconsin–Madison is a public research university located in Madison, Wisconsin, United States. Founded in 1848, UW–Madison is the flagship campus of the University of Wisconsin System. It became a land-grant institution in 1866...
. In one study a stable silylene is synthesized by debromination the dibromosilyl compound 1 by potassium graphite:
The α-nitrogen atoms in 2 stabilize the electrophilic silicon atom by п-donation. In one contributing resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
structure 2b a negative charge is centered in an exocyclic methylene group and a positive charge delocalized over the silicon ring system. X-ray diffraction of the compound shows equal N-Si bonds (173 pm) and an unusually short exocylic bond with bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of 143.6 pm. The compound reacts with trimethylsilyltriflate to the 1,4-adduct 3 (kinetic product) which slowly isomerizes to the 1,1-adduct 4 as the thermodynamically controlled
Thermodynamic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity...
product.