
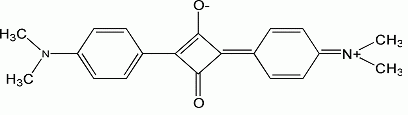
Squaraine dye
Encyclopedia

Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, typically in the red and near infrared region (absorption maxima are found between 630 and 670 nm and their emission maxima are between 650–700 nm). They are characterized by their unique aromatic four membered ring system derived from squaric acid
Squaric acid
Squaric acid, also called quadratic acid, because its four carbon atoms approximately form a square, is an organic compound with chemical formula 424....
. Most squaraines are wikt:encumbered by nucleophilic attack of the central four membered ring, which is highly electron deficient. This encumbrance can be attenuated by the formation of a rotaxane
Rotaxane
A rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
around the dye to protect it from nucleophiles. They are currently used as sensors for ions and have recently, with the advent of protected squanaine derivatives, been exploited in biomedical imaging.
Synthesis
Synthesis of squaraine dyes was reported at least in 1966. They are derived from squaric acid which undergoes an electrophilic aromatic substitutionElectrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
reaction with an aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
or another electron rich derivative to form a highly conjugated product with extensive charge distribution.
For instance, squaraine dyes are also formed via reaction of squaric acid
Squaric acid
Squaric acid, also called quadratic acid, because its four carbon atoms approximately form a square, is an organic compound with chemical formula 424....
or its derivatives with so-called "methylene bases" like 2-methyl-indolenines, 2-methyl-benzthiazoles or 2-methyl-benzo-selenazoles. Indolenine-based squaraines combine good photostability including high quantum yields when bound to proteins and reactive versions of these dyes are commonly used as fluorescent probes and labels for biomedical applications.
Squarylium dye III
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
and a few others. Their absorption peaks at ~630 nm and luminescence at ~650 nm. The luminescence is photochemically stable and its quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
is ~0.65.
Squarylium dye molecules can be encapsulated into carbon nanotubes enhancing the optical properties of carbon nanotubes
Optical properties of carbon nanotubes
Within materials science, the optical properties of carbon nanotubes refer specifically to the absorption, photoluminescence, and Raman spectroscopy of carbon nanotubes. Spectroscopic methods offer the possibility of quick and non-destructive characterization of relatively large amounts of carbon...
. Efficient energy transfer occurs between the encapsulated dye and nanotube — light is absorbed by the dye and without significant loss is transferred to the nanotubes. Encapsulation increases chemical and thermal stability of squarylium molecules; it also allows their isolation and individual characterization. For example, encapsulation of dye molecules inside carbon nanotubes completely quenches strong dye luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
, thus allowing measurement and analysis of their Raman
Raman scattering
Raman scattering or the Raman effect is the inelastic scattering of a photon. It was discovered by Sir Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan in liquids, and by Grigory Landsberg and Leonid Mandelstam in crystals....
spectra.

