Steric effects
Encyclopedia
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
within a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
due to overlapping electron clouds (Pauli
Wolfgang Pauli
Wolfgang Ernst Pauli was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after being nominated by Albert Einstein, he received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or...
or Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
repulsion), and this may affect the molecule's preferred shape (conformation
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
) and reactivity
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
.
Steric hindrance
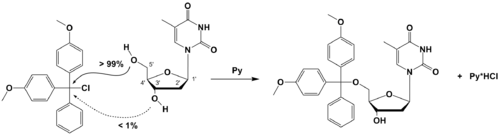
Steric hindrance or steric resistance occurs when the size of groups within a molecule prevents chemical reactions that are observed in related smaller molecules. Although steric hindrance is sometimes a problem (it prevents SN2 reactionsSN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
with tertiary substrates from taking place), it can also be a very useful tool, and is often exploited by chemists to change the reactivity pattern of a molecule by stopping unwanted side-reactions (steric protection). Steric hindrance between adjacent groups can also restrict torsional bond angles
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
. However, hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
has been suggested as an explanation for the preference of the staggered conformation
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
of ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
because the steric hindrance of the small hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom is far too small. This is the effect responsible for the observed shape of rotaxane
Rotaxane
A rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
s.
Other types of steric effects
Steric shielding occurs when a chargedElectric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
group on a molecule is seemingly weakened or spatially shielded by less charged (or oppositely charged) atoms, including counterions
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
in solution (Debye
Peter Debye
Peter Joseph William Debye FRS was a Dutch physicist and physical chemist, and Nobel laureate in Chemistry.-Early life:...
shielding). In some cases, for an atom to interact with sterically shielded atoms, it would have to approach from a vicinity where there is less shielding, thus controlling where and from what direction a molecular interaction can take place.
Steric attraction occurs when molecules have shapes or geometries that are optimized for interaction with one another. In these cases molecules will react with each other most often in specific arrangements.
Chain crossing: A chain
Random coil
A random coil is a polymer conformation where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules...
, ring, or a set of rings cannot change from one conformation to another if it would require a chain (or ring - a ring is a cyclic chain) to pass through itself or another chain. This is responsible for the shape of catenane
Catenane
A catenane is a mechanically-interlocked molecular architecture consisting of two or more interlocked macrocycles. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin catena meaning "chain"...
s and molecular knot
Molecular knot
In chemistry, a molecular knot, or knotane, is a mechanically-interlocked molecular architecture that is analogous to a macroscopic knot. A molecular knot in a trefoil knot configuration is chiral, having at least two enantiomers. Examples of naturally formed knotanes are DNA and certain proteins....
s.
Steric repulsions between different parts of molecular system were found of key importance to govern the direction of transition metal mediated transformations and catalysis. Steric effect can even induce a mechanism switch in the catalytic reaction.
Steric effects vs. electronic effects
The structure, properties, and reactivity of a molecule is dependent on straight forward bonding interactions including covalent bondCovalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s, ionic bond
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
s, hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s and lesser forms of bonding. This bonding supplies a basic molecular skeleton that is modified by repulsive forces. These repulsive forces include the steric interactions described above. Basic bonding and steric are at times insufficient to explain many structures, properties, and reactivity. Thus steric effects are often contrasted and complemented by electronic effects implying the influence of effects such as induction, conjunction, orbital symmetry, electrostatic interactions, and spin state. There are more esoteric electronic effects but these are among the most important when considering structure and chemical reactivity.
A special computational procedure was developed to separate electronic and steric effects of an arbitrary group in the molecule and to reveal their influence on structure and reactivity.
Significance
Understanding steric effects is critical to chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
and pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
. In chemistry, steric effects are nearly universal and affect the rates and energies of most chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s to varying degrees. In biochemistry, steric effects are often exploited in naturally occurring molecules such as enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s, where the catalytic
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
site may be buried within a large protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
structure. In pharmacology, steric effects determine how and at what rate a drug
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
will interact with its target bio-molecules.
See also
- Collision theoryCollision theoryCollision theory is a theory proposed by Max Trautz and William Lewis in 1916 and 1918, that qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total...
- Reaction rate acceleration as result of steric hindrance in the Thorpe-Ingold effectThorpe-Ingold effectThe Thorpe–Ingold effect or gem-dimethyl effect, or angle compression is an effect observed in organic chemistry where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents...
- Sterically induced reductionSterically induced reductionIn chemistry, a sterically induced reduction happens when an oxidized metal behaves and exhibits similar if not identical reducing properties as that of the more reduced form of the metal...

