
Super resolution microscopy
Encyclopedia
Super-resolution microscopy is a form of light microscopy
. Due to the diffraction of light
, the resolution
of conventional light microscopy is limited as stated by Ernst Abbe in 1873. A good approximation of the resolution attainable is the FWHM (full width at half-maximum) of the point spread function
, and a precise widefield microscope with high numerical aperture and visible light usually reaches a resolution of ~250 nm.
Super-resolution techniques allow the capture of images with a higher resolution than the diffraction limit. They fall into two broad categories, "true" super-resolution techniques, which capture information contained in evanescent waves, and "functional" super-resolution techniques, which uses clever experimental techniques and known limitations on the matter being imaged to reconstruct a super-resolution image.
True subwavelength imaging techniques include those that utilize the Pendry Superlens
and Near field scanning optical microscopy, the 4Pi Microscope
and structured illumination microscopy technologies like SIM and SMI
. However, the majority of techniques of importance in biological imaging fall into the functional category.
There are two major groups of methods for functional super-resolution microscopy:
as a confocal laser scanning fluorescence microscope where the light is focused ideally from all sides to a common focus that is used to scan the object by 'point-by-point' excitation combined with 'point-by-point' detection.
Some of the following information was gathered (with permission) from a chemistry blog's review of sub-diffraction microscopy techniques Part I and Part II. For a review, see also reference.
of the aperture in the far-field. But, in the near-field, all of this is not necessarily the case. Near-field scanning optical microscopy (NSOM) forces light through the tiny tip of a pulled fiber — and the aperture can be on the order of tens of nanometers. When the tip is brought to nanometers away from a molecule, the resolution is limited not by diffraction but by the size of the tip aperture (because only that one molecule will see the light coming out of the tip). An image can be built by a raster scan
of the tip over the surface to create an image.
The main down-side to NSOM is the limited number of photons you can force out a tiny tip, and the minuscule collection efficiency (if one is trying to collect fluorescence in the near-field). Other techniques such as ANSOM (see below) try to avoid this drawback.
Bowtie nanoantennas have been used to greatly and reproducibly enhance the electric field in the nanometer gap between the tips two gold triangles. Again, the point is to enhance a very small region of a diffraction-limited spot, thus improving the mismatch between light and nanoscale objects—and breaking the diffraction barrier.
is a laser scanning fluorescence microscope
with an improved axial
resolution
. The typical value of 500-700 nm can be improved to 100-150 nm, which corresponds to an almost spherical focal spot with 5-7 times less volume than that of standard confocal microscopy
.
The improvement in resolution is achieved by using two opposing objective lenses both of which focused to the same geometrical location. Also the difference in optical path length
through each of the two objective lenses is carefully aligned to be minimal. By this, molecules residing in the common focal area of both objectives can be illuminated coherently from both sides and also the reflected or emitted light can be collected coherently, i.e. coherent superposition of emitted light on the detector is possible. The solid angle
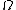 that is used for illumination and detection is increased and approaches the ideal case. In this case the sample is illuminated and detected from all sides simultaneously.
that is used for illumination and detection is increased and approaches the ideal case. In this case the sample is illuminated and detected from all sides simultaneously.
Up to now, the best quality in a 4Pi microscope was reached in conjunction with the STED principle.
 There is also the wide-field structured-illumination (SI) approach to breaking the diffraction limit of light. SI—or patterned illumination—relies on both specific microscopy protocols and extensive software analysis post-exposure. But, because SI is a wide-field technique, it is usually able to capture images at a higher rate than confocal-based schemes like STED (but SI is not actually superfast). The main concept of SI is to illuminate a sample with patterned light and increase the resolution by measuring the fringes in the Moiré pattern
There is also the wide-field structured-illumination (SI) approach to breaking the diffraction limit of light. SI—or patterned illumination—relies on both specific microscopy protocols and extensive software analysis post-exposure. But, because SI is a wide-field technique, it is usually able to capture images at a higher rate than confocal-based schemes like STED (but SI is not actually superfast). The main concept of SI is to illuminate a sample with patterned light and increase the resolution by measuring the fringes in the Moiré pattern
(from the interference of the illumination pattern and the sample). "Otherwise-unobservable sample information can be deduced from the fringes and computationally restored."
SI enhances spatial resolution by collecting information from frequency space outside the observable region. This process is done in reciprocal space: The Fourier transform
(FT) of an SI image contains superimposed additional information from different areas of reciprocal space; with several frames with the illumination shifted by some phase, it is possible to computationally separate and reconstruct the FT image, which has much more resolution information. The reverse FT returns the reconstructed image to a super-resolution image.
Spatially Modulated Illumination (SMI
SMI microscopy is a light optical process of the so-called Point Spread Function
-Engineering. These are processes that modify the Point Spread Function (PSF) of a microscope
in a suitable manner to either increase the optical resolution, to maximize the precision of distance
measurements of fluorescent objects that are small relative to the wavelength
of the illuminating light, or to extract other structural parameters in the nanometer range.
The Vertico SMI
microscope achieves this in the following manner: The illumination intensity within the object range is not uniform, unlike conventional wide field fluorescence microscopes, but is spatially modulated in a precise manner by the use of one or two opposing interfering laser beams along the axis. The object is moved in high-precision steps through the wave field, or the wave field itself is moved relative to the object by phase shift. This results in an improved axial size and distance resolution.
. Within RESOLFT the principle of STED microscopy
and GSD microscopy
are generalized.
s by means of stimulated emission
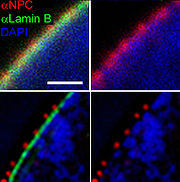
. In practice, the excitation laser pulse is first applied whereby a STED pulse soon follows (But STED without pulses using continuous wave lasers is also used). Furthermore, the STED pulse is modified in such a way that it features a zero-intensity spot, which coincides with the excitation focal spot. Due to the non-linear dependence of the stimulated emission rate on the intensity of the STED beam, all the fluorophores around the focal excitation spot will be in their off state (the ground state of the fluorophores). By scanning this focal spot one retrieves the image. The FWHM of the PSF of the excitation focal spot can theoretically be compressed to an arbitrary width by raising the intensity of the STED pulse, according to equation .
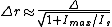
∆r is the lateral resolution, ∆ is the FWHM of the diffraction limited PSF, Imax is the peak intensity of the STED laser, and Is is the threshold intensity needed in order to achieve saturated emission depletion.
The main disadvantage of STED, which has prevented its widespread use, is that the machinery is complicated. On the one hand, the image acquisition speed is relatively slow for large fields of view because of the need to scan the sample in order to retrieve an image. On the other hand, it can be very fast for smaller fields of view: Recordings with up to 80 frames per second have been shown.
Due to a large Is value associated with STED, there is a need for a high-intensity excitation pulse, which may cause damage to the sample.
or Ground State Depletion microscopy, uses the triplet state
of a fluorophore as the off-state and the singlet state as the on-state, whereby an excitation laser is used to drive the fluorophores at the periphery of the singlet state molecule to the triplet state. This is much like STED, where the off-state is the ground state of fluorophores, which is why equation also applies in this case. The Is value is smaller than in STED making super-resolution imaging possible at a much smaller laser intensity. Compared to STED though, the fluorophores used in GSD are generally less photostable and the saturation of the triplet state may be harder to realize.
SSIM requires shifting the illumination pattern multiple times, effectively limiting the temporal resolution of the technique. In addition there is a need of very photostable fluorophores due to the saturating conditions. These conditions also induce radiation damage to the sample that restricts the possible applications in which SSIM may be used.
SSIM section merged from Super-resolution microscopy page
Structured illumination microscopy only enhances the resolution only by a factor of 2 (because the SI pattern cannot be focused to anything smaller than half the wavelength of the excitation light). To further increase the resolution, one can introduce nonlinearities, which show up as higher-order harmonics in the FT. In reference, Gustafsson uses saturation of the fluorescent sample as the nonlinear effect. A sinusoidal saturating excitation beam produces the distorted fluorescence intensity pattern in the emission. This nonpolynomial nonlinearity yields a series of higher-order harmonics in the FT.
Each higher-order harmonic in the FT allows another set of images that can be used to reconstruct a larger area in reciprocal space, and thus a higher resolution. In this case, Gustafsson achieves less than 50-nm resolving power, more than five times that of the microscope in its normal configuration.
The main problems with SI are that, in this incarnation, saturating excitation powers cause more photodamage and lower fluorophore photostability, and sample drift must be kept to below the resolving distance. The former limitation might be solved by using a different nonlinearity (such as stimulated emission depletion or reversible photoactivation, both of which are used in other sub-diffraction imaging schemes); the latter limits live-cell imaging and may require faster frame rates or the use of some fiduciary marker
s for drift subtraction. Nevertheless, SI is certainly a strong contender for further application in the field of super-resolution microscopy.
Examples for this microscopy are shown under 2.4 SIM (3D-SIM Microscopy)
Normally, the width of the point spread function (~ 250 nm) limits resolution. However, given an isolated emitter, one is able to determine its location with a precision only limited by its intensity according to equation .
Here, Δloc is the localization precision, Δ is the FWHM of the PSF and N is the number of collected photons.
This fitting process can only be performed reliably for isolated emitters (see Deconvolution
), and interesting biological samples are so densely labeled with emitters that fitting is impossible when all emitters are active at the same time.
SMLM techniques solve this dilemma by activating only a sparse subset of emitters at the same time, localizing these few emitters very precisely, deactivating them and activating another subset.
Generally, localization microscopy is performed with fluorophores. Suitable fluorophores reside in a non-fluorescent dark state for most of the time and are activated stochastically, most of the time with an excitation laser of low intensity. A readout laser stimulates fluorescence and bleaches or photoswitches the fluorophores back to a dark state, typically within 10-100 ms. The photons emitted during the fluorescent phase are collected with a camera and the
resulting image of the fluorophore (which is distorted by the PSF) can be fitted with very high precision. Repeating the process several thousand times ensures that all fluorophores can go through the bright state and are recorded. A computer then reconstructs a super-resolved image.
The desirable traits of fluorophores used for these methods, in order to maximize the resolution, are that they should be bright. That is, they should have a high extinction coefficient
and a high quantum yield
. They should also possess a high contrast ratio (ratio between the number of photons emitted in the light state and the number of photons emitted in the dark state). Also, a densely labeled sample is desirable according to the Nyquist criteria.
The multitude of localization microscopy methods differ mostly in the type of fluorophores used.
"Optically isolated" means that at a given point in time, only a single particle/molecule within a region of a size determined by conventional optical resolution (typically approx. 200-250 nm diameter
) is being registered. This is possible when molecules within such a region all carry different spectral markers (e.g. different colors or other usable differences in the light emission of different particles).
The structural resolution achievable using SPDM can be expressed in terms of the smallest measurable distance between two in their spatial position determined punctiform particle of different spectral characteristics ("topological resolution“). Modeling has shown that under suitable conditions regarding the precision of localization, particle density etc., the "topological resolution" corresponds to a "space frequency," which in terms of the classical definition is equivalent to a much-improved optical resolution.
SPDM is a localization microscopy that achieves an effective optical resolution several times better than the conventional optical resolution, represented by the half-width of the main maximum of the effective point image function. By applying suitable laser optical precision processes, position and distances can be measured with nanometer accuracy between targets with different spectral signatures.
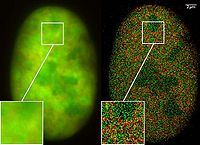 Localization microscopy for many standard fluorescent dyes like GFP
Localization microscopy for many standard fluorescent dyes like GFP
, Alexa dyes and fluorescein molecules is possible if certain photo-physical conditions are present. With this so-called SPDMphymod (physically modifiable fluorophores) technology a single laser wavelength of suitable intensity is sufficient for nanoimaging in contrast to other localization microscopy technologies that need two laser wavelengths when special photo-switchable/photo-activatable fluorescence molecules are used.
Based on singlet triplet state transitions it is crucial for SPDMphymod that this process is ongoing and leading to the effect that a single molecule comes first into a very long-living reversible dark state (with half-life of several seconds even) from which it returns to a fluorescent state emitting many photons for several milliseconds before it returns into a very long-living so-called irreversible dark state. SPDMphymod microscopy uses fluorescent molecules that are emitting the same spectral light frequency but with different spectral signatures based on the flashing characteristics. By combining two thousands images of the same cell, it is possible using laser optical precision measurements to record localization images with significantly improved optical resolution.
Standard fluorescent dyes already successfully used with the SPDMphymod technology are GFP, RFP, YFP, Alexa 488, Alexa 568, Alexa 647, Cy2, Cy3, Atto 488 and fluorescein.
STORM has also been extended to three-dimensional imaging using optical astigmatism, in which the elliptical shape of the point spread function encodes the x, y, and z positions for samples up to several micrometers thick, and has been demonstrated in living cells. To date, the spatial resolution achieved by this technique is ~20 nm in the lateral dimensions and ~50 nm in the axial dimension and the temporal resolution is as fast as ~0.5 - 1s.
In dSTORM, fluorophores are embedded in an oxidizing and reducing buffer system (ROXS) and fluorescence is excited. Sometimes, stochastically, the fluorophore will enter a
triplet state, in which it is susceptible to the reducing components in the buffer. The fluorophore is reduced into a long-lived radical state, which is dark for several
seconds.
are made possible by the combination of SMI
and SPDM, whereby first the SMI and then the SPDM process is applied.
The SMI
process determines the center of particles and their spread in the direction of the microscope axis. While the center of particles/molecules can be determined with a 1-2 nm precision, the spread around this point can be determined down to an axial diameter of approx. 30-40 nm.
Subsequently, the lateral position of the individual particle/molecule is determined using SPDM, achieving a precision of a few nanometers.
As a biological application in the 3D dual color mode the spatial arrangements of Her2/neu and Her3 clusters was achieved. The positions in all three directions of the protein clusters could be determined with an accuracy of about 25 nm.
Microscopy
Microscopy is the technical field of using microscopes to view samples and objects that cannot be seen with the unaided eye...
. Due to the diffraction of light
Diffraction
Diffraction refers to various phenomena which occur when a wave encounters an obstacle. Italian scientist Francesco Maria Grimaldi coined the word "diffraction" and was the first to record accurate observations of the phenomenon in 1665...
, the resolution
Optical resolution
Optical resolution describes the ability of an imaging system to resolve detail in the object that is being imaged.An imaging system may have many individual components including a lens and recording and display components...
of conventional light microscopy is limited as stated by Ernst Abbe in 1873. A good approximation of the resolution attainable is the FWHM (full width at half-maximum) of the point spread function
Point spread function
The point spread function describes the response of an imaging system to a point source or point object. A more general term for the PSF is a system's impulse response, the PSF being the impulse response of a focused optical system. The PSF in many contexts can be thought of as the extended blob...
, and a precise widefield microscope with high numerical aperture and visible light usually reaches a resolution of ~250 nm.
Super-resolution techniques allow the capture of images with a higher resolution than the diffraction limit. They fall into two broad categories, "true" super-resolution techniques, which capture information contained in evanescent waves, and "functional" super-resolution techniques, which uses clever experimental techniques and known limitations on the matter being imaged to reconstruct a super-resolution image.
True subwavelength imaging techniques include those that utilize the Pendry Superlens
Superlens
A superlens, super lens or perfect lens is a lens which uses metamaterials to go beyond the diffraction limit. The diffraction limit is an inherent limitation in conventional optical devices or lenses. In 2000, a type of lens was proposed, consisting of a metamaterial that compensates for wave...
and Near field scanning optical microscopy, the 4Pi Microscope
4Pi Microscope
A 4Pi Microscope is a laser scanning fluorescence microscope with an improved axial resolution. The typical value of 500-700 nm can be improved to 100-150 nm which corresponds to an almost spherical focal spot with 5-7 times less volume than that of standard confocal microscopy.-Working...
and structured illumination microscopy technologies like SIM and SMI
SMI
SMI may refer to:Business:*Snow Machines, Inc., a Michigan based snowmaking equipment manufacturer.*Supplier Managed Inventory, a business model*Swiss Market Index, Switzerland's blue-chip indexCompanies:...
. However, the majority of techniques of importance in biological imaging fall into the functional category.
There are two major groups of methods for functional super-resolution microscopy:
- Deterministic super-resolution: The most commonly used emitters in biological microscopy, fluorophores, show a nonlinear response to excitation, and this nonlinear response can be exploited to enhance resolution. These methods include STEDSTED microscopyStimulated Emission Depletion microscopy, or STED microscopy, is a fluorescence microscopy technique that uses the non-linear de-excitation of fluorescent dyes to overcome the resolution limit imposed by diffraction with standard confocal laser scanning microscopes and conventional far-field...
, GSDGSD microscopyGround State Depletion Microscopy, or GSD Microscopy, is an implementation of the RESOLFT concept. The method was proposed in 1995 and experimentally demonstrated in 2007. It is the second concept to overcome the diffraction barrier in far-field optical microscopy published by Stefan Hell...
, RESOLFTRESOLFTRESOLFT, an acronym for REversible Saturable OpticaL Fluorescence Transitions, denotes a group of optical microscopy techniques with very high resolution...
and SSIM.
- Stochastical super-resolution: The chemical complexity of many molecular light sources gives them a complex temporal behaviour, which can be used to make several close-by fluorophores emit light at separate times and thereby become resolvable in time. These methods include SOFI and all single-molecule localization methods (SMLM) such as SPDM, SPDMphymod,PALM, FPALM, STORM and dSTORM.
History
In 1978, the first theoretical ideas had been developed to break this barrier using a 4Pi Microscope4Pi Microscope
A 4Pi Microscope is a laser scanning fluorescence microscope with an improved axial resolution. The typical value of 500-700 nm can be improved to 100-150 nm which corresponds to an almost spherical focal spot with 5-7 times less volume than that of standard confocal microscopy.-Working...
as a confocal laser scanning fluorescence microscope where the light is focused ideally from all sides to a common focus that is used to scan the object by 'point-by-point' excitation combined with 'point-by-point' detection.
Some of the following information was gathered (with permission) from a chemistry blog's review of sub-diffraction microscopy techniques Part I and Part II. For a review, see also reference.
Near-field scanning optical microscope (NSOM)
Near-field scanning is also called NSOM. Probably the most conceptual way to break the diffraction barrier is to use a light source and/or a detector that is itself nanometer in scale. Diffraction as we know it is truly a far-field effect: The light from an aperture is the Fourier transformFourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
of the aperture in the far-field. But, in the near-field, all of this is not necessarily the case. Near-field scanning optical microscopy (NSOM) forces light through the tiny tip of a pulled fiber — and the aperture can be on the order of tens of nanometers. When the tip is brought to nanometers away from a molecule, the resolution is limited not by diffraction but by the size of the tip aperture (because only that one molecule will see the light coming out of the tip). An image can be built by a raster scan
Raster scan
A raster scan, or raster scanning, is the rectangular pattern of image capture and reconstruction in television. By analogy, the term is used for raster graphics, the pattern of image storage and transmission used in most computer bitmap image systems...
of the tip over the surface to create an image.
The main down-side to NSOM is the limited number of photons you can force out a tiny tip, and the minuscule collection efficiency (if one is trying to collect fluorescence in the near-field). Other techniques such as ANSOM (see below) try to avoid this drawback.
Local enhancement / ANSOM / optical nano-antennas
Instead of forcing photons down a tiny tip, some techniques create a local bright spot in an otherwise diffraction-limited spot. ANSOM is apertureless NSOM: it uses a tip very close to a fluorophore to enhance the local electric field the fluorophore sees. Basically, the ANSOM tip is like a lightning rod, which creates a hot spot of light.Bowtie nanoantennas have been used to greatly and reproducibly enhance the electric field in the nanometer gap between the tips two gold triangles. Again, the point is to enhance a very small region of a diffraction-limited spot, thus improving the mismatch between light and nanoscale objects—and breaking the diffraction barrier.
4Pi
A 4Pi Microscope4Pi Microscope
A 4Pi Microscope is a laser scanning fluorescence microscope with an improved axial resolution. The typical value of 500-700 nm can be improved to 100-150 nm which corresponds to an almost spherical focal spot with 5-7 times less volume than that of standard confocal microscopy.-Working...
is a laser scanning fluorescence microscope
Fluorescence microscope
A fluorescence microscope is an optical microscope used to study properties of organic or inorganic substances using the phenomena of fluorescence and phosphorescence instead of, or in addition to, reflection and absorption...
with an improved axial
Optical axis
An optical axis is a line along which there is some degree of rotational symmetry in an optical system such as a camera lens or microscope.The optical axis is an imaginary line that defines the path along which light propagates through the system...
resolution
Optical resolution
Optical resolution describes the ability of an imaging system to resolve detail in the object that is being imaged.An imaging system may have many individual components including a lens and recording and display components...
. The typical value of 500-700 nm can be improved to 100-150 nm, which corresponds to an almost spherical focal spot with 5-7 times less volume than that of standard confocal microscopy
Confocal microscopy
Confocal microscopy is an optical imaging technique used to increase optical resolution and contrast of a micrograph by using point illumination and a spatial pinhole to eliminate out-of-focus light in specimens that are thicker than the focal plane. It enables the reconstruction of...
.
The improvement in resolution is achieved by using two opposing objective lenses both of which focused to the same geometrical location. Also the difference in optical path length
Optical path length
In optics, optical path length or optical distance is the product of the geometric length of the path light follows through the system, and the index of refraction of the medium through which it propagates. A difference in optical path length between two paths is often called the optical path...
through each of the two objective lenses is carefully aligned to be minimal. By this, molecules residing in the common focal area of both objectives can be illuminated coherently from both sides and also the reflected or emitted light can be collected coherently, i.e. coherent superposition of emitted light on the detector is possible. The solid angle
Solid angle
The solid angle, Ω, is the two-dimensional angle in three-dimensional space that an object subtends at a point. It is a measure of how large that object appears to an observer looking from that point...
 that is used for illumination and detection is increased and approaches the ideal case. In this case the sample is illuminated and detected from all sides simultaneously.
that is used for illumination and detection is increased and approaches the ideal case. In this case the sample is illuminated and detected from all sides simultaneously.Up to now, the best quality in a 4Pi microscope was reached in conjunction with the STED principle.
Structured illumination microscopy (SIM)

Moiré pattern
In physics, a moiré pattern is an interference pattern created, for example, when two grids are overlaid at an angle, or when they have slightly different mesh sizes.- Etymology :...
(from the interference of the illumination pattern and the sample). "Otherwise-unobservable sample information can be deduced from the fringes and computationally restored."
SI enhances spatial resolution by collecting information from frequency space outside the observable region. This process is done in reciprocal space: The Fourier transform
Fourier transform
In mathematics, Fourier analysis is a subject area which grew from the study of Fourier series. The subject began with the study of the way general functions may be represented by sums of simpler trigonometric functions...
(FT) of an SI image contains superimposed additional information from different areas of reciprocal space; with several frames with the illumination shifted by some phase, it is possible to computationally separate and reconstruct the FT image, which has much more resolution information. The reverse FT returns the reconstructed image to a super-resolution image.
Spatially Modulated Illumination (SMISMISMI may refer to:Business:*Snow Machines, Inc., a Michigan based snowmaking equipment manufacturer.*Supplier Managed Inventory, a business model*Swiss Market Index, Switzerland's blue-chip indexCompanies:...
)
SMI microscopy is a light optical process of the so-called Point Spread FunctionPoint spread function
The point spread function describes the response of an imaging system to a point source or point object. A more general term for the PSF is a system's impulse response, the PSF being the impulse response of a focused optical system. The PSF in many contexts can be thought of as the extended blob...
-Engineering. These are processes that modify the Point Spread Function (PSF) of a microscope
Microscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
in a suitable manner to either increase the optical resolution, to maximize the precision of distance
Distance
Distance is a numerical description of how far apart objects are. In physics or everyday discussion, distance may refer to a physical length, or an estimation based on other criteria . In mathematics, a distance function or metric is a generalization of the concept of physical distance...
measurements of fluorescent objects that are small relative to the wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of the illuminating light, or to extract other structural parameters in the nanometer range.
The Vertico SMI
Vertico SMI
Vertico-SMI is currently the fastest light microscope for the 3D analysis of complete cells in the nanometer range. It is based on two technologies developed in 1996, SMI and SPDM...
microscope achieves this in the following manner: The illumination intensity within the object range is not uniform, unlike conventional wide field fluorescence microscopes, but is spatially modulated in a precise manner by the use of one or two opposing interfering laser beams along the axis. The object is moved in high-precision steps through the wave field, or the wave field itself is moved relative to the object by phase shift. This results in an improved axial size and distance resolution.
Deterministic functional techniques
RESOLFT microscopy is an optical microscopy with very high resolution that can image details in samples that cannot be imaged with conventional or confocal microscopyConfocal microscopy
Confocal microscopy is an optical imaging technique used to increase optical resolution and contrast of a micrograph by using point illumination and a spatial pinhole to eliminate out-of-focus light in specimens that are thicker than the focal plane. It enables the reconstruction of...
. Within RESOLFT the principle of STED microscopy
STED microscopy
Stimulated Emission Depletion microscopy, or STED microscopy, is a fluorescence microscopy technique that uses the non-linear de-excitation of fluorescent dyes to overcome the resolution limit imposed by diffraction with standard confocal laser scanning microscopes and conventional far-field...
and GSD microscopy
GSD microscopy
Ground State Depletion Microscopy, or GSD Microscopy, is an implementation of the RESOLFT concept. The method was proposed in 1995 and experimentally demonstrated in 2007. It is the second concept to overcome the diffraction barrier in far-field optical microscopy published by Stefan Hell...
are generalized.
Stimulated emission depletion (STED)
STED (Stimulated Emission Depletion microscopy) uses two laser pulses, the excitation pulse for excitation of the fluorophores to their fluorescent state and the STED pulse for the de-excitation of fluorophoreFluorophore
A fluorophore, in analogy to a chromophore, is a component of a molecule which causes a molecule to be fluorescent. It is a functional group in a molecule which will absorb energy of a specific wavelength and re-emit energy at a different wavelength...
s by means of stimulated emission
Stimulated emission
In optics, stimulated emission is the process by which an atomic electron interacting with an electromagnetic wave of a certain frequency may drop to a lower energy level, transferring its energy to that field. A photon created in this manner has the same phase, frequency, polarization, and...
. In practice, the excitation laser pulse is first applied whereby a STED pulse soon follows (But STED without pulses using continuous wave lasers is also used). Furthermore, the STED pulse is modified in such a way that it features a zero-intensity spot, which coincides with the excitation focal spot. Due to the non-linear dependence of the stimulated emission rate on the intensity of the STED beam, all the fluorophores around the focal excitation spot will be in their off state (the ground state of the fluorophores). By scanning this focal spot one retrieves the image. The FWHM of the PSF of the excitation focal spot can theoretically be compressed to an arbitrary width by raising the intensity of the STED pulse, according to equation .
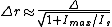
∆r is the lateral resolution, ∆ is the FWHM of the diffraction limited PSF, Imax is the peak intensity of the STED laser, and Is is the threshold intensity needed in order to achieve saturated emission depletion.
The main disadvantage of STED, which has prevented its widespread use, is that the machinery is complicated. On the one hand, the image acquisition speed is relatively slow for large fields of view because of the need to scan the sample in order to retrieve an image. On the other hand, it can be very fast for smaller fields of view: Recordings with up to 80 frames per second have been shown.
Due to a large Is value associated with STED, there is a need for a high-intensity excitation pulse, which may cause damage to the sample.
Ground state depletion (GSD)
GSD microscopyGSD microscopy
Ground State Depletion Microscopy, or GSD Microscopy, is an implementation of the RESOLFT concept. The method was proposed in 1995 and experimentally demonstrated in 2007. It is the second concept to overcome the diffraction barrier in far-field optical microscopy published by Stefan Hell...
or Ground State Depletion microscopy, uses the triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
of a fluorophore as the off-state and the singlet state as the on-state, whereby an excitation laser is used to drive the fluorophores at the periphery of the singlet state molecule to the triplet state. This is much like STED, where the off-state is the ground state of fluorophores, which is why equation also applies in this case. The Is value is smaller than in STED making super-resolution imaging possible at a much smaller laser intensity. Compared to STED though, the fluorophores used in GSD are generally less photostable and the saturation of the triplet state may be harder to realize.
Spatially Structured Illumination Microscopy (SSIM)
SSIM (Spatially Structured Illumination microscopy) exploits the nonlinear dependence of the emission rate of fluorophores on the intensity of the excitation laser. By applying a sinusoidal illumination pattern with a peak intensity close to that needed in order to saturate the fluorophores in their fluorescent state one retrieves moiré fringes. The fringes contain high order spatial information that may be extracted by computational techniques. Once the information is extracted a super-resolution image is retrieved.SSIM requires shifting the illumination pattern multiple times, effectively limiting the temporal resolution of the technique. In addition there is a need of very photostable fluorophores due to the saturating conditions. These conditions also induce radiation damage to the sample that restricts the possible applications in which SSIM may be used.
SSIM section merged from Super-resolution microscopy page
Structured illumination microscopy only enhances the resolution only by a factor of 2 (because the SI pattern cannot be focused to anything smaller than half the wavelength of the excitation light). To further increase the resolution, one can introduce nonlinearities, which show up as higher-order harmonics in the FT. In reference, Gustafsson uses saturation of the fluorescent sample as the nonlinear effect. A sinusoidal saturating excitation beam produces the distorted fluorescence intensity pattern in the emission. This nonpolynomial nonlinearity yields a series of higher-order harmonics in the FT.
Each higher-order harmonic in the FT allows another set of images that can be used to reconstruct a larger area in reciprocal space, and thus a higher resolution. In this case, Gustafsson achieves less than 50-nm resolving power, more than five times that of the microscope in its normal configuration.
The main problems with SI are that, in this incarnation, saturating excitation powers cause more photodamage and lower fluorophore photostability, and sample drift must be kept to below the resolving distance. The former limitation might be solved by using a different nonlinearity (such as stimulated emission depletion or reversible photoactivation, both of which are used in other sub-diffraction imaging schemes); the latter limits live-cell imaging and may require faster frame rates or the use of some fiduciary marker
Fiduciary marker
A fiducial marker or fiducial is an object used in the field of view of an imaging system which appears in the image produced, for use as a point of reference or a measure...
s for drift subtraction. Nevertheless, SI is certainly a strong contender for further application in the field of super-resolution microscopy.
Examples for this microscopy are shown under 2.4 SIM (3D-SIM Microscopy)
Localization Microscopy
Single-molecule localization microscopy (SMLM) summarizes all microscopical techniques that achieve super-resolution by isolating emitters and fitting their images with the point spread function (PSF).Normally, the width of the point spread function (~ 250 nm) limits resolution. However, given an isolated emitter, one is able to determine its location with a precision only limited by its intensity according to equation .
Here, Δloc is the localization precision, Δ is the FWHM of the PSF and N is the number of collected photons.
This fitting process can only be performed reliably for isolated emitters (see Deconvolution
Deconvolution
In mathematics, deconvolution is an algorithm-based process used to reverse the effects of convolution on recorded data. The concept of deconvolution is widely used in the techniques of signal processing and image processing...
), and interesting biological samples are so densely labeled with emitters that fitting is impossible when all emitters are active at the same time.
SMLM techniques solve this dilemma by activating only a sparse subset of emitters at the same time, localizing these few emitters very precisely, deactivating them and activating another subset.
Generally, localization microscopy is performed with fluorophores. Suitable fluorophores reside in a non-fluorescent dark state for most of the time and are activated stochastically, most of the time with an excitation laser of low intensity. A readout laser stimulates fluorescence and bleaches or photoswitches the fluorophores back to a dark state, typically within 10-100 ms. The photons emitted during the fluorescent phase are collected with a camera and the
resulting image of the fluorophore (which is distorted by the PSF) can be fitted with very high precision. Repeating the process several thousand times ensures that all fluorophores can go through the bright state and are recorded. A computer then reconstructs a super-resolved image.
The desirable traits of fluorophores used for these methods, in order to maximize the resolution, are that they should be bright. That is, they should have a high extinction coefficient
Mass attenuation coefficient
The mass attenuation coefficient is a measurement of how strongly a chemical species or substance absorbs or scatters light at a given wavelength, per unit mass...
and a high quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
. They should also possess a high contrast ratio (ratio between the number of photons emitted in the light state and the number of photons emitted in the dark state). Also, a densely labeled sample is desirable according to the Nyquist criteria.
The multitude of localization microscopy methods differ mostly in the type of fluorophores used.
SPDM: Localization Microscopy
SPDM (Spectral Precision Distance Microscopy), the first described localization microscopy technology 1997 is a light optical process of fluorescence microscopy, which allows position, distance and angle measurements on "optically isolated" particles (e.g. molecules) well below the theoretical limit of resolution for light microscopy."Optically isolated" means that at a given point in time, only a single particle/molecule within a region of a size determined by conventional optical resolution (typically approx. 200-250 nm diameter
Diameter
In geometry, a diameter of a circle is any straight line segment that passes through the center of the circle and whose endpoints are on the circle. The diameters are the longest chords of the circle...
) is being registered. This is possible when molecules within such a region all carry different spectral markers (e.g. different colors or other usable differences in the light emission of different particles).
The structural resolution achievable using SPDM can be expressed in terms of the smallest measurable distance between two in their spatial position determined punctiform particle of different spectral characteristics ("topological resolution“). Modeling has shown that under suitable conditions regarding the precision of localization, particle density etc., the "topological resolution" corresponds to a "space frequency," which in terms of the classical definition is equivalent to a much-improved optical resolution.
SPDM is a localization microscopy that achieves an effective optical resolution several times better than the conventional optical resolution, represented by the half-width of the main maximum of the effective point image function. By applying suitable laser optical precision processes, position and distances can be measured with nanometer accuracy between targets with different spectral signatures.
SPDMphymod

Green fluorescent protein
The green fluorescent protein is a protein composed of 238 amino acid residues that exhibits bright green fluorescence when exposed to blue light. Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the...
, Alexa dyes and fluorescein molecules is possible if certain photo-physical conditions are present. With this so-called SPDMphymod (physically modifiable fluorophores) technology a single laser wavelength of suitable intensity is sufficient for nanoimaging in contrast to other localization microscopy technologies that need two laser wavelengths when special photo-switchable/photo-activatable fluorescence molecules are used.
Based on singlet triplet state transitions it is crucial for SPDMphymod that this process is ongoing and leading to the effect that a single molecule comes first into a very long-living reversible dark state (with half-life of several seconds even) from which it returns to a fluorescent state emitting many photons for several milliseconds before it returns into a very long-living so-called irreversible dark state. SPDMphymod microscopy uses fluorescent molecules that are emitting the same spectral light frequency but with different spectral signatures based on the flashing characteristics. By combining two thousands images of the same cell, it is possible using laser optical precision measurements to record localization images with significantly improved optical resolution.
Standard fluorescent dyes already successfully used with the SPDMphymod technology are GFP, RFP, YFP, Alexa 488, Alexa 568, Alexa 647, Cy2, Cy3, Atto 488 and fluorescein.
Photoactivated localization microscopy (PALM) and FPALM
PALM is single-molecule localization microscopy with fluorescent proteins. FPALM is the same method published concurrently under the same name.Stochastic optical reconstruction microscopy (STORM)
STORM is a super-resolution imaging technique that utilizes sequential activation and localization of photoswitchable fluorophores to create high resolution images. During imaging, only an optically resolvable subset of fluorophores is activated to a fluorescent state at any given instant, such that the position of each fluorophore can be determined with high precision by finding the centroid positions of the single-molecule images of these fluorophores. The fluorophores are subsequently deactivated, and another subset is activated and imaged. Iterating this process allows numerous fluorophores to be localized and a super-resolution image to be constructed from these localizations. In principle any photoswitchable fluorophore can be used, and STORM has been demonstrated with a variety of different probes and labeling strategies. Using stochastic photoswitching of single fluorophores, such as Cy5, STORM can be performed with a single red laser excitation source. The red laser both switches the Cy5 fluorophore to a dark state by formation of an adduct and subsequently returns the molecule to the fluorescent state. Many other dyes can also be used for STORM. In addition to single fluorophores, dye-pairs consisting of an activator fluorophore (such as Alexa 405, Cy2, and Cy3) and a photoswitchable reporter dye (such as Cy5, Alexa 647, Cy5.5, and Cy7) can be used for STORM. In this scheme, the activator fluorophore, when excited near its absorption maximum, serves to reactivate the photoswitchable dye to the fluorescent state. Multicolor imaging has been performed by using different activation wavelengths to distinguish dye-pairs based on the activator fluorophore used or using spectrally distinct photoswitchable fluorophores either with or without activator fluorophores. In addition to organic dyes, photoswitchable fluorescent proteins can also be used. Highly specific labeling of biological structures with photoswitchable probes has been achieved with antibody staining, direct conjugation of proteins, and genetic encoding.STORM has also been extended to three-dimensional imaging using optical astigmatism, in which the elliptical shape of the point spread function encodes the x, y, and z positions for samples up to several micrometers thick, and has been demonstrated in living cells. To date, the spatial resolution achieved by this technique is ~20 nm in the lateral dimensions and ~50 nm in the axial dimension and the temporal resolution is as fast as ~0.5 - 1s.
Direct stochastical optical reconstruction microscopy (dSTORM)
dSTORM utilizes the photoswitching of a single fluorophore.In dSTORM, fluorophores are embedded in an oxidizing and reducing buffer system (ROXS) and fluorescence is excited. Sometimes, stochastically, the fluorophore will enter a
triplet state, in which it is susceptible to the reducing components in the buffer. The fluorophore is reduced into a long-lived radical state, which is dark for several
seconds.
Software for localization microscopy
Localization microscopy depends heavily on software that can precisely fit the point spread function (PSF) to millions of images of active fluorophores within a few minutes. Since the classical analysis methods and software suites used in natural sciences are too slow to computationally solve these problems, often taking hours of computation for processing data measured in minutes, specialized software programs have been developed.- rapidSTORM is currently (2011) the largest and most evolved software suite available.
Super-resolution Optical Fluctuation Imaging (SOFI)
It is possible to circumvent the need for PSF fitting inherent in single molecule localization microscopy (SMLM) by directly computing the temporal autocorrelation of pixels. This technique is called super-resolution optical fluctuation imaging (SOFI) and has been shown to be more precise than SMLM when the density of concurrently active fluorophores is very high.3D Light microscopical nanosizing (LIMON) microscopy
3 D LIMON (Light MicrOscopical nanosizing microscopy) images using Vertico SMIVertico SMI
Vertico-SMI is currently the fastest light microscope for the 3D analysis of complete cells in the nanometer range. It is based on two technologies developed in 1996, SMI and SPDM...
are made possible by the combination of SMI
SMI
SMI may refer to:Business:*Snow Machines, Inc., a Michigan based snowmaking equipment manufacturer.*Supplier Managed Inventory, a business model*Swiss Market Index, Switzerland's blue-chip indexCompanies:...
and SPDM, whereby first the SMI and then the SPDM process is applied.
The SMI
SMI
SMI may refer to:Business:*Snow Machines, Inc., a Michigan based snowmaking equipment manufacturer.*Supplier Managed Inventory, a business model*Swiss Market Index, Switzerland's blue-chip indexCompanies:...
process determines the center of particles and their spread in the direction of the microscope axis. While the center of particles/molecules can be determined with a 1-2 nm precision, the spread around this point can be determined down to an axial diameter of approx. 30-40 nm.
Subsequently, the lateral position of the individual particle/molecule is determined using SPDM, achieving a precision of a few nanometers.
As a biological application in the 3D dual color mode the spatial arrangements of Her2/neu and Her3 clusters was achieved. The positions in all three directions of the protein clusters could be determined with an accuracy of about 25 nm.

