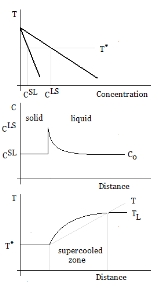
Supercooling
Encyclopedia
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid
or a gas
below its freezing point
without it becoming a solid
.
A liquid below its standard freezing point will crystallize in the presence of a seed crystal or nucleus
around which a crystal
structure can form. However, lacking any such nucleus
, the liquid phase
can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs. The homogeneous nucleation can occur above the glass transition where the system is an amorphous (non-crystalline) solid.
Pure water
normally freezes at 273.15 K
(0 °C or 32 °F) but it can also be "supercooled" at standard pressure down to its crystal homogeneous nucleation at almost 224.8 K (−48.3 °C/−55 °F). If, however, it is cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 165 K (−108 °C/−162.4 °F).
Glassy water
can be heated up to approximately 150 K (−123 °C/−189.4 °F).
In the range of temperatures between 231 K (−42 °C/−43.6 °F) and 150 K (−123 °C/−189.4 °F) experiments find only crystal ice.
Droplets of supercooled water often exist in stratiform
and cumulus
cloud
s. Aircraft
flying through these clouds seed an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft
's wings or blockage of its instruments and probes, unless the aircraft are equipped with an appropriate de-icing system. Freezing rain
is also caused by supercooled droplets.
The process opposite to supercooling, the melting of a solid above the freezing point, is much more difficult, and a solid will almost always melt at the same temperature
for a given pressure
. For this reason, it is the melting point which is usually identified, using melting point apparatus
; even when the subject of a paper is "freezing-point determination", the actual methodology is "the principle of observing the disappearance rather than the formation of ice". It is, however, possible, at a given pressure to superheat
a liquid above its boiling point
without it becoming gaseous.
Supercooling is often confused with freezing-point depression
. Supercooling is the cooling of a liquid below its freezing point without it becoming solid. Freezing point depression is when a solution
can be cooled below the freezing point of the corresponding pure liquid due to the presence of the solute
; an example of this is the freezing point depression that occurs when sodium chloride
is added to pure water.
and vacuole
and thereby survive temperatures down to −40 °C. This is partly achieved through the synthesis of antifreeze protein
s that prevent ice nucleation.
Source: "Teleost fish have an osmotic concentration in their body fluids of about 300–400 mosm
, and this corresponds to a freezing point of about −0.6 to −0.8 °C. Sea water in the polar regions often has a temperature of about −1,8 °C ... Do they have a lower freezing point than ordinary fish, or do they remain supercooled throughout life? The answer is that both possibilities seem to have been realized.", Knut Schmidt-Nielsen, Animal Physiology, Adaption and Environment (Cambridge University Press, 1975), p.279.
"In summer, the surface fish in the Hebron Fjord, Labrador, have no freezing problem. The fish that live deeper, however, where the water is at −1.73 °C, have a freezing point in their body fluids of −1.0 °C and must remain supercooled (Scholander et al., 1957)." (Ibid., p. 280)
. For example, there are freezers that cool drinks to a supercooled level so that when it is opened it slushes over. Another example is a product that can supercool the beverage in a conventional freezer. The Coca-Cola Company
also briefly marketed special vending machines containing Sprite
in the UK, and Coke in Singapore, which stored the bottles in a supercooled state so that they would turn to slush upon opening.
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
below its freezing point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
without it becoming a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
.
A liquid below its standard freezing point will crystallize in the presence of a seed crystal or nucleus
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
around which a crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
structure can form. However, lacking any such nucleus
Seed crystal
A seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
, the liquid phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs. The homogeneous nucleation can occur above the glass transition where the system is an amorphous (non-crystalline) solid.
Pure water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
normally freezes at 273.15 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
(0 °C or 32 °F) but it can also be "supercooled" at standard pressure down to its crystal homogeneous nucleation at almost 224.8 K (−48.3 °C/−55 °F). If, however, it is cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 165 K (−108 °C/−162.4 °F).
Glassy water
Amorphous ice
Amorphous ice is an amorphous solid form of water, meaning it consists of water molecules that are randomly arranged like the atoms of common glass. Everyday ice is a crystalline material where the atoms are regularly arranged in a lattice whereas amorphous ice is distinguished by a lack of...
can be heated up to approximately 150 K (−123 °C/−189.4 °F).
In the range of temperatures between 231 K (−42 °C/−43.6 °F) and 150 K (−123 °C/−189.4 °F) experiments find only crystal ice.
Droplets of supercooled water often exist in stratiform
Stratus cloud
A stratus cloud is a cloud belonging to a class characterized by horizontal layering with a uniform base, as opposed to convective clouds that are as tall or taller than wide . More specifically, the term stratus is used to describe flat, hazy, featureless clouds of low altitude varying in color...
and cumulus
Cumulus cloud
Cumulus clouds are a type of cloud with noticeable vertical development and clearly defined edges. Cumulus means "heap" or "pile" in Latin. They are often described as "puffy" or "cotton-like" in appearance. Cumulus clouds may appear alone, in lines, or in clusters...
cloud
Cloud
A cloud is a visible mass of liquid droplets or frozen crystals made of water and/or various chemicals suspended in the atmosphere above the surface of a planetary body. They are also known as aerosols. Clouds in Earth's atmosphere are studied in the cloud physics branch of meteorology...
s. Aircraft
Aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air, or, in general, the atmosphere of a planet. An aircraft counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engines.Although...
flying through these clouds seed an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft
Aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air, or, in general, the atmosphere of a planet. An aircraft counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engines.Although...
's wings or blockage of its instruments and probes, unless the aircraft are equipped with an appropriate de-icing system. Freezing rain
Freezing rain
Freezing rain is the name given to rain that falls when surface temperatures are below freezing. The raindrops become supercooled while passing through a sub-freezing layer of air, many hundred feet , just above the surface, and then freeze upon impact with any object they encounter. The resulting...
is also caused by supercooled droplets.
The process opposite to supercooling, the melting of a solid above the freezing point, is much more difficult, and a solid will almost always melt at the same temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
for a given pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
. For this reason, it is the melting point which is usually identified, using melting point apparatus
Melting point apparatus
A melting point apparatus is a scientific instrument used to determine the melting point of a substance. Some types of melting point apparatuses include the Thiele tube, Fisher-Johns apparatus, Gallenkamp melting point apparatus and automatic melting point apparatus.- Design :While the outward...
; even when the subject of a paper is "freezing-point determination", the actual methodology is "the principle of observing the disappearance rather than the formation of ice". It is, however, possible, at a given pressure to superheat
Superheating
In physics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling...
a liquid above its boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
without it becoming gaseous.
Supercooling is often confused with freezing-point depression
Freezing-point depression
Freezing-point depression describes the phenomenon in which the freezing point of a liquid is depressed when another compound is added, meaning that a solution has a lower freezing point than a pure solvent. This happens whenever a non-volatile solute is added to a pure solvent, such as water...
. Supercooling is the cooling of a liquid below its freezing point without it becoming solid. Freezing point depression is when a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
can be cooled below the freezing point of the corresponding pure liquid due to the presence of the solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
; an example of this is the freezing point depression that occurs when sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
is added to pure water.
In plants
Some plants are able to supercool the fluid in their cells cytosolCytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
and vacuole
Vacuole
A vacuole is a membrane-bound organelle which is present in all plant and fungal cells and some protist, animal and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water containing inorganic and organic molecules including enzymes in solution, though in certain...
and thereby survive temperatures down to −40 °C. This is partly achieved through the synthesis of antifreeze protein
Antifreeze protein
Antifreeze proteins or ice structuring proteins refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be...
s that prevent ice nucleation.
In fish
The osmotic concentration of the body fluids of fish is lower that the osmotic concentration of sea water. Therefore the freezing point of fish can be above (!) the temperature of sea water. Of course, the freezing point can be lowered by anti-freeze agents. But there are fish, whose freezing point is indeed higher than the temperature of the surrounding sea water, and therefore the body fluids of these fish are supercooled. These fish must live well below the water surface, because they must not get in contact with ice (otherwise they would freeze immediately , since they are only supercooled.).Source: "Teleost fish have an osmotic concentration in their body fluids of about 300–400 mosm
Osmole (unit)
Osmolarity is the measure of solute concentration, defined as the number of osmoles of solute per litre of solution . The osmolarity of a solution is usually expressed as Osm/L , in the same way that the molarity of a solution is expressed as "M"...
, and this corresponds to a freezing point of about −0.6 to −0.8 °C. Sea water in the polar regions often has a temperature of about −1,8 °C ... Do they have a lower freezing point than ordinary fish, or do they remain supercooled throughout life? The answer is that both possibilities seem to have been realized.", Knut Schmidt-Nielsen, Animal Physiology, Adaption and Environment (Cambridge University Press, 1975), p.279.
"In summer, the surface fish in the Hebron Fjord, Labrador, have no freezing problem. The fish that live deeper, however, where the water is at −1.73 °C, have a freezing point in their body fluids of −1.0 °C and must remain supercooled (Scholander et al., 1957)." (Ibid., p. 280)
Applications
One commercial application of supercooling is in refrigerationRefrigeration
Refrigeration is a process in which work is done to move heat from one location to another. This work is traditionally done by mechanical work, but can also be done by magnetism, laser or other means...
. For example, there are freezers that cool drinks to a supercooled level so that when it is opened it slushes over. Another example is a product that can supercool the beverage in a conventional freezer. The Coca-Cola Company
The Coca-Cola Company
The Coca-Cola Company is an American multinational beverage corporation and manufacturer, retailer and marketer of non-alcoholic beverage concentrates and syrups. The company is best known for its flagship product Coca-Cola, invented in 1886 by pharmacist John Stith Pemberton in Columbus, Georgia...
also briefly marketed special vending machines containing Sprite
Sprite
sprite or SPRITE may refer to:* any meaning of "spirit"** a ghost, the soul or spirit of a deceased person or animal that can appear, in visible form or other manifestation, to the living...
in the UK, and Coke in Singapore, which stored the bottles in a supercooled state so that they would turn to slush upon opening.
See also
- Amorphous solidAmorphous solidIn condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
- Pumpable ice technologyPumpable ice technologyPumpable ice technology is a technology to produce fluids or secondary refrigerants also called coolants with a viscosity of water or jelly and the cooling capacity of ice...
- SlushSlush (beverage)A slush is a flavored frozen drink.There are a number of different kinds of slush drinks:* Frozen carbonated beverages, typified by the Slurpee or ICEE, are made by freezing a carbonated drink. These machines are complicated and expensive, and notably require a carbon dioxide supply...
- SubcoolingSubcoolingIn refrigeration, subcooling is the process by which a saturated liquid refrigerant is cooled below the saturation temperature, forcing it to change its phase completely. The resulting fluid is called a subcooled liquid and is the convenient state in which refrigerants may undergo the remaining...

