
Thiocyanate
Encyclopedia
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid
. Common derivatives include the colourless salts potassium thiocyanate
and sodium thiocyanate
. Organic compound
s containing the functional group
SCN are also called thiocyanates. Mercury(II) thiocyanate
was formerly used in pyrotechnics.
Thiocyanate is analogous to the cyanate
ion, [OCN]−, wherein oxygen
is replaced by sulfur
. [SCN]− is one of the pseudohalide
s, due to the similarity of its reactions to that of halide
ions. Thiocyanate used to be known as rhodanide (from a Greek
word for rose
) because of the red colour of its complexes with iron
. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate
with cyanide
:
The second reaction is catalyzed by the enzyme sulfotransferase
known as rhodanase
and may be relevant to detoxification of cyanide in the body.
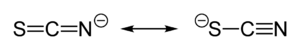 Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. As a consequence, thiocyanate can act as a nucleophile
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. As a consequence, thiocyanate can act as a nucleophile
at either sulfur or nitrogen — it is an ambidentate ligand. [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). Experimental evidence leads to the general conclusion that class A metals
(hard acids
) tend to form N-bonded thiocyanate complexes, whereas class B metals
(soft acids
) tend to form S-bonded thiocyanate complexes. Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2.
s, the substituent
is attached to nitrogen: R−N=C=S has a S-C double bond and a C-N double bond:
 Organic thiocyanates are hydrolyzed to thiocarbamate
Organic thiocyanates are hydrolyzed to thiocarbamate
s in the Riemschneider thiocarbamate synthesis
.
(Fe3+), a blood red solution is formed due to the formation of [Fe(NCS)(H2O)5]2+
.
by a lactoperoxidase
. Thus the complete absence of thiocyanate or reducted thiocyanate in the human body, (e.g., cystic fibrosis
) is of high importance in the human host defense system.
Thiocyanate is a metabolite
of sodium nitroprusside
, after rhodanese catalyses its reaction with thiosulfate
.
Thiocyanic acid
Thiocyanic acid is a chemical compound with the formula HSCN that exists as a mixture with the isomeric compound isothiocyanic acid . It is the sulfur analog of cyanic acid ....
. Common derivatives include the colourless salts potassium thiocyanate
Potassium thiocyanate
Potassium thiocyanate is the chemical compound with the molecular formula KSCN. It is an important salt of the thiocyanate anion, one of the pseudohalides...
and sodium thiocyanate
Sodium thiocyanate
Sodium thiocyanate is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals...
. Organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s containing the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
SCN are also called thiocyanates. Mercury(II) thiocyanate
Mercury(II) thiocyanate
Mercury thiocyanate is an inorganic chemical compound, the salt of Hg2+ and the thiocyanate anion. It is a stable solid at room temperature that has the appearance of white powder with chunks; it can also be grey in color, depending on purity. Mercury compounds are extremely toxic and protective...
was formerly used in pyrotechnics.
Thiocyanate is analogous to the cyanate
Cyanate
The cyanate ion is an anion with the chemical formula written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair...
ion, [OCN]−, wherein oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
is replaced by sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. [SCN]− is one of the pseudohalide
Pseudohalogen
Pseudo'halogen molecules are inorganic molecules of the general formsPs–Ps or Ps–X, where Ps is a pseudohalogen group such as cyanide, cyanate, thiocyanate and others, and X is a "true" halogen...
s, due to the similarity of its reactions to that of halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
ions. Thiocyanate used to be known as rhodanide (from a Greek
Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
word for rose
Rose
A rose is a woody perennial of the genus Rosa, within the family Rosaceae. There are over 100 species. They form a group of erect shrubs, and climbing or trailing plants, with stems that are often armed with sharp prickles. Flowers are large and showy, in colours ranging from white through yellows...
) because of the red colour of its complexes with iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
with cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
:
- 8 CN− + S8 → 8 SCN−
- CN− + S2O32− → SCN− + SO32−
The second reaction is catalyzed by the enzyme sulfotransferase
Sulfotransferase
Sulfotransferases are transferase enzymes that catalyze the transfer of a sulfate group from a donor molecule to an acceptor alcohol or amine. The most common sulfate donor is 3'-phosphoadenosine-5'-phosphosulfate...
known as rhodanase
Rhodanase
Rhodanese is a mitochondrial enzyme that detoxifies cyanide by converting it to thiocyanate .This reaction takes place in two steps. The diagram on the right shows the crystallographically-determined structure of rhodanese. In the first step, thiosulfate reacts with the thiol group on Cysteine-247...
and may be relevant to detoxification of cyanide in the body.
Structure, bonding and coordination chemistry

Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
at either sulfur or nitrogen — it is an ambidentate ligand. [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). Experimental evidence leads to the general conclusion that class A metals
Classes of metals
Class A metals are metals that form hard acids. Hard acids are acids with relatively ionic bonds. These metals, such as iron, aluminum, titanium, sodium, calcium and the lanthanides, would rather bond with fluorine than iodine. They form stable products with hard bases, which are bases with ionic...
(hard acids
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
) tend to form N-bonded thiocyanate complexes, whereas class B metals
Classes of metals
Class A metals are metals that form hard acids. Hard acids are acids with relatively ionic bonds. These metals, such as iron, aluminum, titanium, sodium, calcium and the lanthanides, would rather bond with fluorine than iodine. They form stable products with hard bases, which are bases with ionic...
(soft acids
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
) tend to form S-bonded thiocyanate complexes. Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2.
Organic thiocyanates
Organic and transition metal derivatives of the thiocyanate ion can exist as "linkage isomers." In thiocyanates, the organic group (or metal ion) is attached to sulfur: R−S−C≡N has a S-C single bond and a C-N triple bond. In isothiocyanateIsothiocyanate
Isothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also...
s, the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
is attached to nitrogen: R−N=C=S has a S-C double bond and a C-N double bond:

Thiocarbamate
Thiocarbamates are a family of organosulfur compounds. There are two isomeric forms of thiocarbamate esters: O-thiocarbamates, ROCNR2, and S-thiocarbamates, RSCNR2...
s in the Riemschneider thiocarbamate synthesis
Riemschneider thiocarbamate synthesis
The Riemschneider thiocarbamate synthesis produces aromatic thiocarbamates starting with the corresponding aromatic thiocyanate.500px|centerThe thiocyanate is treated with sulfuric acid and then hydrolyzed with ice water....
.
Test for iron(III)
If [SCN]− is added to a solution containing iron (III) ionsFerric
Ferric refers to iron-containing materials or compounds. In chemistry the term is reserved for iron with an oxidation number of +3, also denoted iron or Fe3+. On the other hand, ferrous refers to iron with oxidation number of +2, denoted iron or Fe2+...
(Fe3+), a blood red solution is formed due to the formation of [Fe(NCS)(H2O)5]2+
Thiocyanatoiron
Thiocyanatoiron is a complex ion with the chemical formula [FeSCN]2+. It is known to produce a blood red color in solution. This is used as a test for Fe3+ in the laboratory...
.
Biological chemistry of thiocyanate in medicine
Thiocyanate is known to be an important part in the biosynthesis of hypothiocyaniteHypothiocyanite
Hypothiocyanite is the anion [OSCN]- and the conjugate base of hypothiocyanous acid. It is an organic compound part of the thiocyanates as it contains the functional group SCN. It is formed when an oxygen is singly bonded to the thiocyanate group...
by a lactoperoxidase
Lactoperoxidase
Lactoperoxidase is a peroxidase enzyme secreted from mammary, salivary, and other mucosal glands that functions as a natural antibacterial agent. Lactoperoxidase is a member of the heme peroxidase family of enzymes. In humans, lactoperoxidase is encoded by the LPO gene.Lactoperoxidase catalyzes...
. Thus the complete absence of thiocyanate or reducted thiocyanate in the human body, (e.g., cystic fibrosis
Cystic fibrosis
Cystic fibrosis is a recessive genetic disease affecting most critically the lungs, and also the pancreas, liver, and intestine...
) is of high importance in the human host defense system.
Thiocyanate is a metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
of sodium nitroprusside
Sodium nitroprusside
Sodium nitroprusside is the inorganic compound with the formula Na2[Fe5NO]·2H2O. This red-coloured salt, which is often abbreviated SNP, is a potent vasodilator...
, after rhodanese catalyses its reaction with thiosulfate
Sodium thiosulfate
Sodium thiosulfate , also spelled sodium thiosulphate, is a colorless crystalline compound that is more familiar as the pentahydrate, Na2S2O3•5H2O, an efflorescent, monoclinic crystalline substance also called sodium hyposulfite or “hypo.”...
.

