Triphenylmethyl radical
Encyclopedia
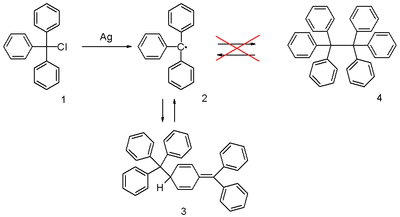
The triphenylmethyl radical is a persistent radical and the first-ever radical
described in organic chemistry
. It can be prepared by homolysis
of triphenylmethyl chloride
1 (scheme 1) by a metal like silver
or zinc
in benzene
or diethyl ether
. The radical 2 forms a chemical equilibrium
with the quinoid type dimer 3. In benzene the concentration of the radical is 2% .
 Solutions containing the radical are yellow
Solutions containing the radical are yellow
and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following Le Chatelier's principle
. Conversely when the solution is cooled it becomes less yellow.
When exposed to air the radical rapidly oxidizes to the peroxide
(Scheme 2) and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine
to triphenylmethyl iodide.
 The radical was discovered by Moses Gomberg
The radical was discovered by Moses Gomberg
in 1900. He tried to prepare hexaphenylethane from triphenylmethyl chloride
and zinc
in benzene
in a Wurtz reaction
and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure 4 in scheme 1 . It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR
data . In hindsight the substituted ethane
molecule does not make sense at all because it is simply too sterically overcrowded.
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
described in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. It can be prepared by homolysis
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
of triphenylmethyl chloride
Triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.-Preparation:Triphenylmethyl chloride is commercially available...
1 (scheme 1) by a metal like silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
or zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
or diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. The radical 2 forms a chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with the quinoid type dimer 3. In benzene the concentration of the radical is 2% .

Yellow
Yellow is the color evoked by light that stimulates both the L and M cone cells of the retina about equally, with no significant stimulation of the S cone cells. Light with a wavelength of 570–590 nm is yellow, as is light with a suitable mixture of red and green...
and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
. Conversely when the solution is cooled it becomes less yellow.
When exposed to air the radical rapidly oxidizes to the peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
(Scheme 2) and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
to triphenylmethyl iodide.

Moses Gomberg
Moses Gomberg was a chemistry professor at the University of Michigan....
in 1900. He tried to prepare hexaphenylethane from triphenylmethyl chloride
Triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.-Preparation:Triphenylmethyl chloride is commercially available...
and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
in a Wurtz reaction
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane which is structure 4 in scheme 1 . It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
data . In hindsight the substituted ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
molecule does not make sense at all because it is simply too sterically overcrowded.

