
Ultraviolet photoelectron spectroscopy
Encyclopedia
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra
of photoelectrons
emitted by molecules which have absorbed ultraviolet
photons, in order to determine molecular energy levels in the valence region.
(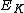 ) of an emitted photoelectron is given by
) of an emitted photoelectron is given by
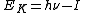 ,
,
where h is Planck’s constant, ν is the frequency of the ionizing light, and I is an ionization energy
corresponding to the energy of an occupied molecular orbital
.
developed X-ray photoelectron spectroscopy
(XPS) for surface chemical analysis. This method uses x-ray sources to study energy levels of atomic core electrons, and at the time had an energy resolution of about 1 eV (electronvolt
).
The ultraviolet method (UPS) was developed to study the photoelectron spectra
of free molecules in the gas phase by David W. Turner
, a physical chemist at Imperial College in London and then at Oxford University, in a series of publications from 1962 to 1967. As a photon source, he used a helium
discharge lamp which emits a wavelength of 58.4 nm (corresponding to an energy of 21.2 eV) in the vacuum ultraviolet region. With this source Turner’s group obtained an energy resolution of 0.02 eV. Turner referred to the method as “molecular photoelectron spectroscopy”, now usually “Ultraviolet photoelectron spectroscopy” or UPS. As compared to XPS, UPS is limited to energy levels of valence electrons, but measures them more accurately. After 1967 commercial UPS spectrometers became available.
energies for comparison with theoretical values from quantum chemistry
, which was also extensively developed in the 1960s. The photoelectron spectrum of a molecule contains a series of peaks each corresponding to one valence-region molecular orbital energy level. Also, the high resolution allowed the observation of fine structure due to vibrational levels of the molecular ion, which facilitates the assignment of peaks to bonding, nonbonding or antibonding molecular orbitals.
The method was later extended to the study of solid surfaces where it is usually described as photoemission spectroscopy
(PES). It is particularly sensitive to the surface region (to 10 nm depth), due to the short range of the emitted photoelectrons (compared to X-rays). It is therefore used to study adsorbed species and their binding to the surface, as well as their orientation on the surface.
A useful result from characterization of solids by UPS is the determination of the work function
of the material. An example of this determination is given by Park et al. Briefly, the full width of the photoelectron spectrum (from the highest kinetic energy/lowest binding energy point to the low kinetic energy cutoff) is measured and subtracted from the photon energy of the exciting radiation, and the difference is the work function. Often, the sample is electrically biased negative to separate the low energy cutoff from the spectrometer response.
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
of photoelectrons
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
emitted by molecules which have absorbed ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
photons, in order to determine molecular energy levels in the valence region.
Basic Theory
If Einstein’s photoelectric law is applied to a free molecule, the kinetic energyKinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
(
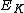 ) of an emitted photoelectron is given by
) of an emitted photoelectron is given by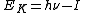 ,
,where h is Planck’s constant, ν is the frequency of the ionizing light, and I is an ionization energy
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
corresponding to the energy of an occupied molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
.
History
Prior to 1960, virtually all measurements of photoelectron kinetic energies were for electrons emitted from metals and other solid surfaces. About 1956 Kai SiegbahnKai Siegbahn
Kai Manne Börje Siegbahn was a Swedish physicist.He was born in Lund, Sweden, and his father Manne Siegbahn also won the Nobel Prize in Physics, in 1924. Siegbahn earned his doctorate at the University of Stockholm in 1944...
developed X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy is a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state of the elements that exist within a material...
(XPS) for surface chemical analysis. This method uses x-ray sources to study energy levels of atomic core electrons, and at the time had an energy resolution of about 1 eV (electronvolt
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
).
The ultraviolet method (UPS) was developed to study the photoelectron spectra
of free molecules in the gas phase by David W. Turner
David W. Turner
David Warren Turner is a physical chemist known for the development of ultra-violet photoelectron spectroscopy , a technique for the measurement of molecular orbital energies in gas-phase molecules....
, a physical chemist at Imperial College in London and then at Oxford University, in a series of publications from 1962 to 1967. As a photon source, he used a helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
discharge lamp which emits a wavelength of 58.4 nm (corresponding to an energy of 21.2 eV) in the vacuum ultraviolet region. With this source Turner’s group obtained an energy resolution of 0.02 eV. Turner referred to the method as “molecular photoelectron spectroscopy”, now usually “Ultraviolet photoelectron spectroscopy” or UPS. As compared to XPS, UPS is limited to energy levels of valence electrons, but measures them more accurately. After 1967 commercial UPS spectrometers became available.
Application
The UPS measures experimental molecular orbitalMolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
energies for comparison with theoretical values from quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
, which was also extensively developed in the 1960s. The photoelectron spectrum of a molecule contains a series of peaks each corresponding to one valence-region molecular orbital energy level. Also, the high resolution allowed the observation of fine structure due to vibrational levels of the molecular ion, which facilitates the assignment of peaks to bonding, nonbonding or antibonding molecular orbitals.
The method was later extended to the study of solid surfaces where it is usually described as photoemission spectroscopy
Photoemission spectroscopy
Photoemission spectroscopy , also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in a substance...
(PES). It is particularly sensitive to the surface region (to 10 nm depth), due to the short range of the emitted photoelectrons (compared to X-rays). It is therefore used to study adsorbed species and their binding to the surface, as well as their orientation on the surface.
A useful result from characterization of solids by UPS is the determination of the work function
Work function
In solid-state physics, the work function is the minimum energy needed to remove an electron from a solid to a point immediately outside the solid surface...
of the material. An example of this determination is given by Park et al. Briefly, the full width of the photoelectron spectrum (from the highest kinetic energy/lowest binding energy point to the low kinetic energy cutoff) is measured and subtracted from the photon energy of the exciting radiation, and the difference is the work function. Often, the sample is electrically biased negative to separate the low energy cutoff from the spectrometer response.

