Valency interaction formula
Encyclopedia
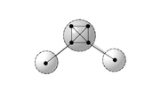
The Valency Interaction Formula, or VIF provides a way of drawing or interpreting the molecular structural formula based on molecular orbital theory
. Valency Points, VP, dots drawn on a page, represent valence orbitals. Valency Interactions, VI, that connect the dots, show interactions between these valence orbitals.
Chemical deductions are made from a VIF picture with the application of two pictorial rules. These are linear transformations applied to the VIF structural formula as a quantum operator. Transformation by the two rules preserves invariants crucial to the characterization of the molecules electronic properties, the numbers of bonding, non-bonding, and anti-bonding orbitals and/or the numbers of doubly, singly, and unoccupied valence orbitals. The two pictorial rules relate all picture with the same electronic properties as characterized by these invariants.
A thorough presentation of VIF is available through the open access journal symmetry.
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
. Valency Points, VP, dots drawn on a page, represent valence orbitals. Valency Interactions, VI, that connect the dots, show interactions between these valence orbitals.
Chemical deductions are made from a VIF picture with the application of two pictorial rules. These are linear transformations applied to the VIF structural formula as a quantum operator. Transformation by the two rules preserves invariants crucial to the characterization of the molecules electronic properties, the numbers of bonding, non-bonding, and anti-bonding orbitals and/or the numbers of doubly, singly, and unoccupied valence orbitals. The two pictorial rules relate all picture with the same electronic properties as characterized by these invariants.
A thorough presentation of VIF is available through the open access journal symmetry.

