
Van Arkel-Ketelaar triangle
Encyclopedia
Anton Eduard van Arkel
Anton Eduard van Arkel, was a Dutch chemist.-See also:*Crystal bar process*Hafnium*Jan Hendrik de Boer*Titanium*Van Arkel-Ketelaar triangle-References:...
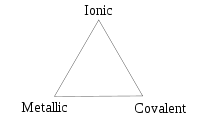
and J. A. A. Ketelaar) are triangles used to show different compounds in varying degrees of ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
, metallic
Metallic bond
Metallic bonding is the electrostatic attractive forces between the delocalized electrons, called conduction electrons, gathered in an "electron sea", and the positively charged metal ions...
and covalent bonding. The bond triangle shows that ionic, metallic and covalent bonds are not just particular bonds of a specific type. Rather, bond types are interconnected and different compounds have varying degrees of different bonding character (for example, covalent bonds with significant ionic character are called polar covalent bonds).
Different compounds can be placed around the triangle. On the right side (from ionic to covalent) should be compounds with varying difference in electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
, in the covalent corner compounds with equal electronegativity such as Cl2 (chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
), in the ionic corner compounds with large electronegativity difference such as NaCl
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
(table salt). The bottom side (from metallic to covalent) is for compounds with varying degree of directionality in the bond. At one extreme is metallic bonds with delocalized bonding and the other are covalent bonds in which the orbitals overlap in a particular direction. The left side (from ionic to metallic) is for delocalized bonds with varying electronegativity difference.
Three species at the vertices of the triangle are: caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
(metallic), fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
(covalent) and caesium fluoride
Caesium fluoride
Caesium fluoride , is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for...
(ionic).

