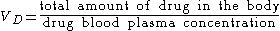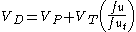Volume of distribution
Encyclopedia
The volume of distribution (VD) , also known as apparent volume of distribution, is a pharmacological
term used to quantify the distribution
of a medication
between plasma
and the rest of the body after oral or parenteral
dosing. It is defined as the theoretical volume in which the total amount of drug would need to be uniformly distributed to produce the desired blood concentration of a drug.
Volume of distribution may be increased by renal failure
(due to fluid retention) and liver failure
(due to altered body fluid and plasma protein binding
). Conversely it may be decreased in dehydration.
The initial volume of distribution
describes blood concentrations prior to attaining the apparent volume of distribution and uses the same formula.
Therefore the dose required to give a certain plasma concentration can be determined if the VD for that drug is known. The VD is not a physiologic value; it is more a reflection of how a drug will distribute throughout the body depending on several physicochemical properties, e.g. solubility, charge, size, etc.
The units for Volume of Distribution are typically reported in (ml or liter)/kg body weight. The fact that VD is a ratio of a theoretical volume to a fixed unit of body weight explains why the VD for children is typically higher than that for adults, even though children are smaller and weigh less. As body composition changes with age, VD decreases.
The VD may also be used to determine how readily a drug will displace into the body tissue compartments relative to the blood:
Where:
If you administer a dose D of a drug intravenously in one go (IV-bolus), you would naively expect it to have an immediate blood concentration which directly corresponds to the amount of blood contained in the body
which directly corresponds to the amount of blood contained in the body  . Mathematically this would be:
. Mathematically this would be:
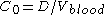
But this is generally not what happens. Instead you observe that the drug has distributed out into some other volume (read organs/tissue). So probably the first question you want to ask is how much of it is no longer in the blood stream?
The volume of distribution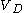 quantifies just that by specifying how big a volume you would need in order to observe the blood concentration actually measured.
quantifies just that by specifying how big a volume you would need in order to observe the blood concentration actually measured.
A practical example for a simple case (mono-compartmental) would be to administer D=8 mg/kg to a human. A human has a blood volume of around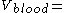 0.08 l/kg
0.08 l/kg
.
This gives a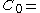 100 µg/ml if the drug stays in the blood stream only, and thus its volume of distribution is the same as
100 µg/ml if the drug stays in the blood stream only, and thus its volume of distribution is the same as 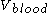 that is
that is 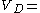 0.08 l/kg. If the drug distributes into all body water the volume of distribution would increase to approximately
0.08 l/kg. If the drug distributes into all body water the volume of distribution would increase to approximately 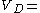 0.57 l/kg
0.57 l/kg
If the drug readily diffuses into the body fat the volume of distribution may increase dramatically, an example is chloroquine
which has a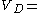 250-302 l/kg
250-302 l/kg
In the simple mono-compartmental case the volume of distribution is defined as: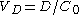 , where the
, where the 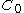 in practice is an extrapolated concentration at time=0 from the first early plasma concentrations after an IV-bolus administration (generally taken around 5min - 30min after giving the drug).
in practice is an extrapolated concentration at time=0 from the first early plasma concentrations after an IV-bolus administration (generally taken around 5min - 30min after giving the drug).
Further reading:
Table of volume of distribution for drugs
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
term used to quantify the distribution
Distribution (pharmacology)
Distribution in pharmacology is a branch of pharmacokinetics which describes the reversible transfer of drug from one location to another within the body....
of a medication
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
between plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
and the rest of the body after oral or parenteral
Parenteral
Parenteral is a route of administration that involves piercing the skin or mucous membrane. Parenteral nutrition refers to providing nutrition via the veins.-Etymology:...
dosing. It is defined as the theoretical volume in which the total amount of drug would need to be uniformly distributed to produce the desired blood concentration of a drug.
Volume of distribution may be increased by renal failure
Renal failure
Renal failure or kidney failure describes a medical condition in which the kidneys fail to adequately filter toxins and waste products from the blood...
(due to fluid retention) and liver failure
Liver failure
Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease , and indicates that the liver has sustained severe damage . The complications are hepatic encephalopathy and impaired protein synthesis...
(due to altered body fluid and plasma protein binding
Plasma protein binding
A drug's efficiency may be affected by the degree to which it binds to the proteins within blood plasma. The less bound a drug is, the more efficiently it can traverse cell membranes or diffuse. Common blood proteins that drugs bind to are human serum albumin, lipoprotein, glycoprotein, α, β‚ and γ...
). Conversely it may be decreased in dehydration.
The initial volume of distribution
Initial volume of distribution
The initial volume of distribution is a pharmacological term used to quantify the distribution of a drug throughout the body relatively soon after oral or intravenous dosing of a drug and prior to the drug reaching a steady state equilibrium. Following distribution of the drug, measurement of blood...
describes blood concentrations prior to attaining the apparent volume of distribution and uses the same formula.
Equations
The volume of distribution is given by the following equation:Therefore the dose required to give a certain plasma concentration can be determined if the VD for that drug is known. The VD is not a physiologic value; it is more a reflection of how a drug will distribute throughout the body depending on several physicochemical properties, e.g. solubility, charge, size, etc.
The units for Volume of Distribution are typically reported in (ml or liter)/kg body weight. The fact that VD is a ratio of a theoretical volume to a fixed unit of body weight explains why the VD for children is typically higher than that for adults, even though children are smaller and weigh less. As body composition changes with age, VD decreases.
The VD may also be used to determine how readily a drug will displace into the body tissue compartments relative to the blood:
Where:
- VP = plasma volume
- VT = apparent tissue volume
- fu = fraction unbound in plasma
- fuT = fraction unbound in tissue
Examples
Understanding volume of distributionIf you administer a dose D of a drug intravenously in one go (IV-bolus), you would naively expect it to have an immediate blood concentration
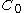 which directly corresponds to the amount of blood contained in the body
which directly corresponds to the amount of blood contained in the body 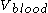 . Mathematically this would be:
. Mathematically this would be: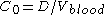
But this is generally not what happens. Instead you observe that the drug has distributed out into some other volume (read organs/tissue). So probably the first question you want to ask is how much of it is no longer in the blood stream?
The volume of distribution
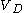 quantifies just that by specifying how big a volume you would need in order to observe the blood concentration actually measured.
quantifies just that by specifying how big a volume you would need in order to observe the blood concentration actually measured.A practical example for a simple case (mono-compartmental) would be to administer D=8 mg/kg to a human. A human has a blood volume of around
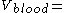 0.08 l/kg
0.08 l/kg.
This gives a
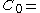 100 µg/ml if the drug stays in the blood stream only, and thus its volume of distribution is the same as
100 µg/ml if the drug stays in the blood stream only, and thus its volume of distribution is the same as 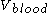 that is
that is 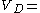 0.08 l/kg. If the drug distributes into all body water the volume of distribution would increase to approximately
0.08 l/kg. If the drug distributes into all body water the volume of distribution would increase to approximately 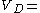 0.57 l/kg
0.57 l/kgIf the drug readily diffuses into the body fat the volume of distribution may increase dramatically, an example is chloroquine
Chloroquine
Chloroquine is a 4-aminoquinoline drug used in the treatment or prevention of malaria.-History:Chloroquine , N'--N,N-diethyl-pentane-1,4-diamine, was discovered in 1934 by Hans Andersag and co-workers at the Bayer laboratories who named it "Resochin". It was ignored for a decade because it was...
which has a
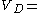 250-302 l/kg
250-302 l/kgIn the simple mono-compartmental case the volume of distribution is defined as:
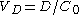 , where the
, where the 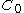 in practice is an extrapolated concentration at time=0 from the first early plasma concentrations after an IV-bolus administration (generally taken around 5min - 30min after giving the drug).
in practice is an extrapolated concentration at time=0 from the first early plasma concentrations after an IV-bolus administration (generally taken around 5min - 30min after giving the drug).Further reading:
Table of volume of distribution for drugs
Table of volume of distribution for drugs
This is a table of volume of distribution for various medication. For comparison, those with a Vd/kg body weight of less than 0.2 are mainly distributed in blood plasma, 0.2-0.7 mostly in the extracellular fluid and those with more than 0.7 are distributed throughout total body water....
| Drug | VD | Comments >- | Warfarin Warfarin Warfarin is an anticoagulant. It is most likely to be the drug popularly referred to as a "blood thinner," yet this is a misnomer, since it does not affect the thickness or viscosity of blood... |
8L | >- | 30L | >- | 15000L | lipophilic Lipophilic Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true... molecules which sequester into total body fat. >- | NXY-059 NXY-059 Disufenton sodium is the disulfonyl derivative of the neuroprotective spin trap phenylbutynitrone or "PBN". It was under development at the drug company AstraZeneca. A 2005 phase-3 clinical trial called "SAINT-1" reported some efficacy in the acute treatment of ischemia injury due to stroke... |
8L | Highly-charged hydrophilic molecule. |