
Acid-base imbalance
Encyclopedia
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma
pH
to deviate out of the normal range (7.35 to 7.45). In the fetus
, the normal range differs based on which umbilical vessel is sampled (umbilical vein
pH is normally 7.25 to 7.45; umbilical artery
pH is normally 7.18 to 7.38). It can exist in varying levels of severity, some life-threatening.
 An excess of acid is called acidosis
An excess of acid is called acidosis
and an excess in bases is called alkalosis
. The process that causes the imbalance is classified based on the etiology
of the disturbance (respiratory or metabolic) and the direction of change in pH (acidosis or alkalosis). This yields the following four basic processes:
in conjunction with respiratory acidosis
. Any combination is possible, except concurrent respiratory acidosis and respiratory alkalosis, since a person cannot breathe too fast and too slow at the same time.
approach. The main variants are the base excess
approach and the bicarbonate
approach. The quantitative
approach introduced by Peter A Stewart
in 1978 is newer.
Sources of acid loss include:
s exist which reversibly bind hydrogen ions and impede any change in pH. Extracellular
buffers include bicarbonate
and ammonia
, while protein
s and phosphate
act as intracellular
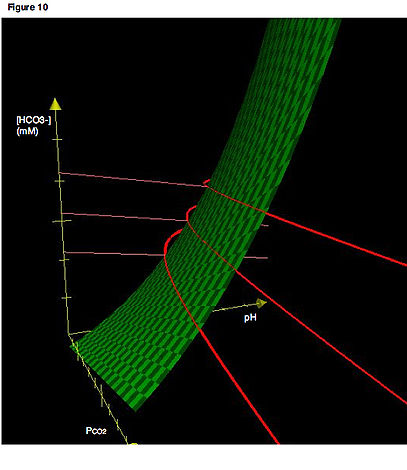
buffers. The bicarbonate buffering system
is especially key, as carbon dioxide
(CO2) can be shifted through carbonic acid
(H2CO3) to hydrogen ions and bicarbonate
(HCO3- ) as shown below.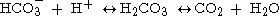
Acid–base imbalances that overcome the buffer system can be compensated in the short term by changing the rate of ventilation
. This alters the concentration of carbon dioxide
in the blood, shifting the above reaction according to Le Chatelier's principle
, which in turn alters the pH. For instance, if the blood pH drops too low (acidemia), the body will compensate by increasing breathing, expelling CO2, and shifting the following reaction to the right such that less hydrogen ions are free - thus the pH will rise back to normal. For alkalemia, the opposite occurs.
The kidneys are slower to compensate, but renal physiology
has several powerful mechanisms to control pH by the excretion of excess acid or base. In responses to acidosis, tubular cells reabsorb more bicarbonate from the tubular fluid, collecting duct cells secrete more hydrogen and generate more bicarbonate, and ammoniagenesis leads to increased formation of the NH3 buffer. In responses to alkalosis, the kidney may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and lowering rates of glutamine
metabolism and ammonia excretion.
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
to deviate out of the normal range (7.35 to 7.45). In the fetus
Fetus
A fetus is a developing mammal or other viviparous vertebrate after the embryonic stage and before birth.In humans, the fetal stage of prenatal development starts at the beginning of the 11th week in gestational age, which is the 9th week after fertilization.-Etymology and spelling variations:The...
, the normal range differs based on which umbilical vessel is sampled (umbilical vein
Umbilical vein
The umbilical vein is a vein present during fetal development that carries oxygenated blood from the placenta to the growing fetus.The blood pressure inside the umbilical vein is approximately 20 mmHg.-Development:...
pH is normally 7.25 to 7.45; umbilical artery
Umbilical artery
The umbilical artery is a paired artery that is found in the abdominal and pelvic regions. In the fetus, it extends into the umbilical cord.-Umbilical arteries in the fetus:...
pH is normally 7.18 to 7.38). It can exist in varying levels of severity, some life-threatening.
Classification

Acidosis
Acidosis is an increased acidity in the blood and other body tissue . If not further qualified, it usually refers to acidity of the blood plasma....
and an excess in bases is called alkalosis
Alkalosis
Alkalosis refers to a condition reducing hydrogen ion concentration of arterial blood plasma . Generally, alkalosis is said to occur when pH of the blood exceeds 7.45. The opposite condition is acidosis .-Causes:...
. The process that causes the imbalance is classified based on the etiology
Etiology
Etiology is the study of causation, or origination. The word is derived from the Greek , aitiologia, "giving a reason for" ....
of the disturbance (respiratory or metabolic) and the direction of change in pH (acidosis or alkalosis). This yields the following four basic processes:
| process | pH PH In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline... |
carbon dioxide Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... |
compensation >- | metabolic acidosis Metabolic acidosis In medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the... |
down | down | >- | down | up | >- | up | up | >- | up | down | renal |
Mixed disorders
The presence of only one of the above derangements is called a simple acid–base disorder. In a mixed disorder more than one is occurring at the same time. Mixed disorders may feature an acidosis and alkosis at the same time that partially counteract each other, or there can be two different conditions effecting the pH in the same direction. The phrase "mixed acidosis", for example, refers to metabolic acidosisMetabolic acidosis
In medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
in conjunction with respiratory acidosis
Respiratory acidosis
Respiratory acidosis is a medical condition in which decreased ventilation causes increased blood carbon dioxide concentration and decreased pH ....
. Any combination is possible, except concurrent respiratory acidosis and respiratory alkalosis, since a person cannot breathe too fast and too slow at the same time.
Calculation of imbalance
The traditional approach to the study of acid–base physiology has been the empiricEmpiric
Empiric can refer to:* Asclepiades of Bithynia* Empiricism* Empirical* Empirical research* Empirical formula...
approach. The main variants are the base excess
Base excess
In human physiology, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L, with positive numbers indicating an excess of base and negative a deficit...
approach and the bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
approach. The quantitative
Quantitative analysis (chemistry)
In chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance present in a sample....
approach introduced by Peter A Stewart
Peter A Stewart
Peter A. Stewart was a Canadian physiologist who introduced an alternate approach to understanding acid base physiology.He outlined his model in a paper in 1978, explained it his 1981 book, How to Understand Acid-Base...
in 1978 is newer.
Causes
There are numerous reasons that each of the four processes can occur (detailed in each article). Generally speaking, sources of acid gain include:- Retention of carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
- Production of nonvolatile acidNonvolatile acidA nonvolatile acid is an acid produced from sources other than carbon dioxide, and is not excreted by the lungs. They are produced from e.g. an incomplete metabolism of carbohydrates, fats, and proteins. All acids produced in the body are nonvolatile except carbonic acid, which is the sole...
s from the metabolism of proteins and other organic molecules - Loss of bicarbonateBicarbonateIn inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
in fecesFecesFeces, faeces, or fæces is a waste product from an animal's digestive tract expelled through the anus or cloaca during defecation.-Etymology:...
or urineUrineUrine is a typically sterile liquid by-product of the body that is secreted by the kidneys through a process called urination and excreted through the urethra. Cellular metabolism generates numerous by-products, many rich in nitrogen, that require elimination from the bloodstream... - Intake of acids or acid precursors
Sources of acid loss include:
- Use of hydrogen ions in the metabolism of various organic anions
- Loss of acid in the vomitus or urineUrineUrine is a typically sterile liquid by-product of the body that is secreted by the kidneys through a process called urination and excreted through the urethra. Cellular metabolism generates numerous by-products, many rich in nitrogen, that require elimination from the bloodstream...
Compensation
The body's acid–base balance is tightly regulated. Several buffering agentBuffering agent
A buffering agent is a weak acid or base used to maintain the acidity of a solution at a chosen value. The function of a buffering agent is to prevent a rapid change in pH when acids or bases are added to the solution. Buffering agents have variable properties—some are more soluble than others;...
s exist which reversibly bind hydrogen ions and impede any change in pH. Extracellular
Extracellular
In cell biology, molecular biology and related fields, the word extracellular means "outside the cell". This space is usually taken to be outside the plasma membranes, and occupied by fluid...
buffers include bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, while protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
act as intracellular
Intracellular
Not to be confused with intercellular, meaning "between cells".In cell biology, molecular biology and related fields, the word intracellular means "inside the cell".It is used in contrast to extracellular...
buffers. The bicarbonate buffering system
Bicarbonate buffering system
The bicarbonate buffering system is an important buffer system in the acid-base homeostasis of living things, including humans. As a buffer, it tends to maintain a relatively constant plasma pH and counteract any force that would alter it....
is especially key, as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) can be shifted through carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
(H2CO3) to hydrogen ions and bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
(HCO3- ) as shown below.
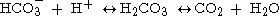
Acid–base imbalances that overcome the buffer system can be compensated in the short term by changing the rate of ventilation
Ventilation (physiology)
In respiratory physiology, ventilation is the rate at which gas enters or leaves the lung. It is categorized under the following definitions:-Sample values:...
. This alters the concentration of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in the blood, shifting the above reaction according to Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
, which in turn alters the pH. For instance, if the blood pH drops too low (acidemia), the body will compensate by increasing breathing, expelling CO2, and shifting the following reaction to the right such that less hydrogen ions are free - thus the pH will rise back to normal. For alkalemia, the opposite occurs.
The kidneys are slower to compensate, but renal physiology
Renal physiology
Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including reabsorption of glucose, amino acids, and other small molecules; regulation of sodium, potassium, and other electrolytes; regulation of fluid balance and blood pressure;...
has several powerful mechanisms to control pH by the excretion of excess acid or base. In responses to acidosis, tubular cells reabsorb more bicarbonate from the tubular fluid, collecting duct cells secrete more hydrogen and generate more bicarbonate, and ammoniagenesis leads to increased formation of the NH3 buffer. In responses to alkalosis, the kidney may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and lowering rates of glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
metabolism and ammonia excretion.
External links
- On-line text at AnaesthesiaMCQ.com
- Overview at kumc.edu
- Overview at mcgill.ca
- http://www.merck.com/mmhe/sec12/ch159/ch159a.html
- Stewart's original text at acidbase.org
- Overview at med.utah.edu
- Overview at anaesthetist.com
- Overview at anst.uu.se
- Tutorial at acid-base.com
- Online acid–base physiology text
- Diagnoses at lakesidepress.com
- Interpretation at nda.ox.ac.uk
- Acid Base Tutorial

