
Allotropes of phosphorus
Encyclopedia
Elemental phosphorus
can exist in several allotropes
; the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus
and atomic phosphorus.
and instability. The molecule is described as consisting of six single P–P bonds. Two different crystalline forms are known. The α form, which is stable under standard conditions, has a body-centered cubic crystal structure. It transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure.
White phosphorus is a transparent wax
y solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen), is highly flammable and pyrophoric
(self-igniting) upon contact with air as well as toxic
(causing severe liver damage
on ingestion and phossy jaw
from chronic ingestion or inhalation). The odour of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "(di)phosphorus pentoxide
", which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and, indeed, it can be stored under water. It is, however, soluble in benzene
, oils, carbon disulfide
, and disulfur dichloride
.
, which is derived from phosphate rock, is heated in an electric or fuel-fired furnace
in the presence of carbon
and silica. Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid
.
 White phosphorus has an appreciable vapour pressure
White phosphorus has an appreciable vapour pressure
at ordinary temperatures. The vapour density
indicates that the vapour is composed of P4 molecules up to about 800 °C. Above that temperature, dissociation into P2
molecules occurs.
It ignites spontaneously in air at about 50 °C, and at much lower temperatures if finely divided. This combustion gives phosphorus (V) oxide:
Because of this property, white phosphorus is used as a weapon.
s.
 Red phosphorus may be formed by heating white phosphorus to 250 °C (482 °F) or by exposing white phosphorus to sunlight
Red phosphorus may be formed by heating white phosphorus to 250 °C (482 °F) or by exposing white phosphorus to sunlight
. Red phosphorus exists as an amorphous network. Upon further heating, the amorphous red phosphorus crystallizes. Red phosphorus does not ignite in air at temperatures below 240 °C, whereas white phosphorus ignites at about 30 °C.
Red phosphorus can be converted to white phosphorus upon heating to 260 °C, as can be seen when one strikes a match.
It is a controlled substance (precursor) in Russia and much of the rest of the former Soviet Union, due to its use in illicit amphetamine production.

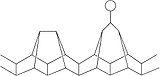
 Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's Metallic Phosphorus. In 1865, Hittorf
Monoclinic phosphorus, or violet phosphorus, is also known as Hittorf's Metallic Phosphorus. In 1865, Hittorf
heated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaque monoclinic, or rhombohedral, crystals sublime. Violet phosphorus can also be prepared by dissolving white phosphorus in molten lead
in a sealed tube at 500 °C for 18 hours. Upon slow cooling, Hittorf's allotrope crystallises
out. The crystals can be revealed by dissolving the lead in dilute nitric acid
followed by boiling in concentrated hydrochloric acid
. In 1865 Johann Wilhelm Hittorf
discovered that when phosphorus was recrystallized from molten lead
, a red/purple form is obtained. This purple form is sometimes known as Hittorf's phosphorus. In addition, a fibrous form exists with similar phosphorus cages. Below is shown a chain of phosphorus atoms which exhibits both the purple and fibrous forms.
and only slowly reacts with halogens. It can be oxidised by nitric acid
to phosphoric acid
.
If it is heated in an atmosphere of inert gas, for example nitrogen
or carbon dioxide
, it sublimes and the vapour condenses as white phosphorus. If, however, it is heated in a vacuum
and the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is a polymer
of high relative molecular mass, which on heating breaks down into P2 molecules. On cooling, these would normally dimerize to give P4 molecules (i.e. white phosphorus) but, in vacuo, they link up again to form the polymeric violet allotrope.
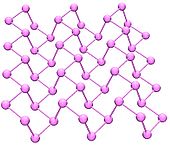
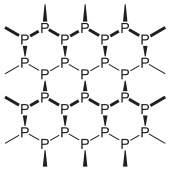 Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure. It is obtained by heating white phosphorus under high pressures (12,000 atmospheres). In appearance, properties and structure it is very like graphite
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure. It is obtained by heating white phosphorus under high pressures (12,000 atmospheres). In appearance, properties and structure it is very like graphite
, being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.
Black phosphorus has an orthorhombic
structure and is the least reactive allotrope: a result of its lattice of interlinked six-membered rings. Each atom is bonded to three other atoms. A recent synthesis of black phosphorus using metal salts as catalysts has been reported.
One of the forms of red/black phosphorus is a cubic
solid.
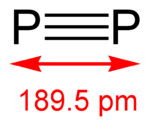 The diphosphorus allotrope (P2) can be obtained normally only under extreme conditions (for example, from P4 at 1100 kelvin). Nevertheless, some advancements were obtained in generating the diatomic molecule in homogenous solution, under normal conditions with the use by some transition metal
The diphosphorus allotrope (P2) can be obtained normally only under extreme conditions (for example, from P4 at 1100 kelvin). Nevertheless, some advancements were obtained in generating the diatomic molecule in homogenous solution, under normal conditions with the use by some transition metal
complexes
(based on for example tungsten
and niobium
).
Diphosphorus is the gaseous form of phosphorus
, and the thermodynamically stable form above 1200 °C and until 2000 °C. The dissociation of tetraphosphorus begins at lower temperature: the percentage of at 800 °C is ≈ 1%. At temperatures about 2000 °C, the diphosphorus molecule begins to dissociate into atomic phosphorus.
s were synthesized as polymers in two modifications.
The red-brown phase differs from red phosphorus and is also stable in air for weeks. Electron microscope
showed the red-brown form as having long, parallel nanorods with a diameter between 0.34 nm
and 0.47 nm.
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
can exist in several allotropes
Allotropy
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
; the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus
Diphosphorus
Diphosphorus, P2, is the diatomic form of phosphorus. Unlike its nitrogen group neighbor nitrogen, which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bonds are high in energy...
and atomic phosphorus.

White phosphorus
White phosphorus, or yellow phosphorus, or simply tetraphosphorus (P4) exists as molecules made up of four atoms. The tetrahedral arrangement results in ring strainRing strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
and instability. The molecule is described as consisting of six single P–P bonds. Two different crystalline forms are known. The α form, which is stable under standard conditions, has a body-centered cubic crystal structure. It transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure.
White phosphorus is a transparent wax
Wax
thumb|right|[[Cetyl palmitate]], a typical wax ester.Wax refers to a class of chemical compounds that are plastic near ambient temperatures. Characteristically, they melt above 45 °C to give a low viscosity liquid. Waxes are insoluble in water but soluble in organic, nonpolar solvents...
y solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenish in the dark (when exposed to oxygen), is highly flammable and pyrophoric
Pyrophoricity
A pyrophoric substance is a substance that will ignite spontaneously in air. Examples are iron sulfide and many reactive metals including uranium, when powdered or sliced thin. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air...
(self-igniting) upon contact with air as well as toxic
Toxicity
Toxicity is the degree to which a substance can damage a living or non-living organisms. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell or an organ , such as the liver...
(causing severe liver damage
Hepatotoxicity
Hepatotoxicity implies chemical-driven liver damage.The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses and sometimes even when introduced within therapeutic ranges, may injure...
on ingestion and phossy jaw
Phossy jaw
Phossy jaw, formally phosphorus necrosis of the jaw, is an occupational disease of those who work with white phosphorus, also known as yellow phosphorus, without proper safeguards. It was most commonly seen in workers in the match industry in the 19th and early 20th century...
from chronic ingestion or inhalation). The odour of combustion of this form has a characteristic garlic smell, and samples are commonly coated with white "(di)phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
", which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and, indeed, it can be stored under water. It is, however, soluble in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, oils, carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, and disulfur dichloride
Disulfur dichloride
Disulfur dichloride is the chemical compound with the formula S2Cl2 . Some alternative names for this compound are sulfur monochloride , disulphur dichloride and sulphur monochloride...
.
Production and applications
The white allotrope can be produced using several different methods. In one process, calcium phosphateCalcium phosphate
Calcium phosphate is the name given to a family of minerals containing calcium ions together with orthophosphates , metaphosphates or pyrophosphates and occasionally hydrogen or hydroxide ions ....
, which is derived from phosphate rock, is heated in an electric or fuel-fired furnace
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
in the presence of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and silica. Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
.

Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
at ordinary temperatures. The vapour density
Vapour density
Vapour density is the density of a vapour in relation to that of hydrogen. It may be defined as mass of a certain volume of a substance divided by mass of same volume of hydrogen....
indicates that the vapour is composed of P4 molecules up to about 800 °C. Above that temperature, dissociation into P2
Diphosphorus
Diphosphorus, P2, is the diatomic form of phosphorus. Unlike its nitrogen group neighbor nitrogen, which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bonds are high in energy...
molecules occurs.
It ignites spontaneously in air at about 50 °C, and at much lower temperatures if finely divided. This combustion gives phosphorus (V) oxide:
- + 5 →
Because of this property, white phosphorus is used as a weapon.
Non-existence of cubic-P8
Although white phosphorus converts to thermodynamically more stable red allotrope, the formation of the cubic P8 is not observed in the condensed phase. Derivatives of this hypothetical molecule have, however, been prepared from phosphaalkynePhosphaalkyne
In chemistry, phosphaalkynes are organophosphorus compounds that have a phosphorus-carbon triple bond....
s.
Red phosphorus

Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
. Red phosphorus exists as an amorphous network. Upon further heating, the amorphous red phosphorus crystallizes. Red phosphorus does not ignite in air at temperatures below 240 °C, whereas white phosphorus ignites at about 30 °C.
Red phosphorus can be converted to white phosphorus upon heating to 260 °C, as can be seen when one strikes a match.
It is a controlled substance (precursor) in Russia and much of the rest of the former Soviet Union, due to its use in illicit amphetamine production.
Hittorf's violet phosphorus



Johann Wilhelm Hittorf
Johann Wilhelm Hittorf was a German physicist who was born in Bonn and died in Münster, Germany.Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules , an important factor in understanding electrochemical reactions...
heated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaque monoclinic, or rhombohedral, crystals sublime. Violet phosphorus can also be prepared by dissolving white phosphorus in molten lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
in a sealed tube at 500 °C for 18 hours. Upon slow cooling, Hittorf's allotrope crystallises
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
out. The crystals can be revealed by dissolving the lead in dilute nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
followed by boiling in concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. In 1865 Johann Wilhelm Hittorf
Johann Wilhelm Hittorf
Johann Wilhelm Hittorf was a German physicist who was born in Bonn and died in Münster, Germany.Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules , an important factor in understanding electrochemical reactions...
discovered that when phosphorus was recrystallized from molten lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
, a red/purple form is obtained. This purple form is sometimes known as Hittorf's phosphorus. In addition, a fibrous form exists with similar phosphorus cages. Below is shown a chain of phosphorus atoms which exhibits both the purple and fibrous forms.
Reactions of violet phosphorus
It does not ignite in air until heated to 300 °C, and it is insoluble in all solvents. It is not attacked by alkaliAlkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
and only slowly reacts with halogens. It can be oxidised by nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
to phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
.
If it is heated in an atmosphere of inert gas, for example nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, it sublimes and the vapour condenses as white phosphorus. If, however, it is heated in a vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
and the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is a polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
of high relative molecular mass, which on heating breaks down into P2 molecules. On cooling, these would normally dimerize to give P4 molecules (i.e. white phosphorus) but, in vacuo, they link up again to form the polymeric violet allotrope.
Black phosphorus


Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.
Black phosphorus has an orthorhombic
Orthorhombic crystal system
In crystallography, the orthorhombic crystal system is one of the seven lattice point groups. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base and height , such that a,...
structure and is the least reactive allotrope: a result of its lattice of interlinked six-membered rings. Each atom is bonded to three other atoms. A recent synthesis of black phosphorus using metal salts as catalysts has been reported.
One of the forms of red/black phosphorus is a cubic
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
solid.
Diphosphorus

Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
complexes
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
(based on for example tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
and niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
).
Diphosphorus is the gaseous form of phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, and the thermodynamically stable form above 1200 °C and until 2000 °C. The dissociation of tetraphosphorus begins at lower temperature: the percentage of at 800 °C is ≈ 1%. At temperatures about 2000 °C, the diphosphorus molecule begins to dissociate into atomic phosphorus.
Phosphorus nanorods
Phosphorus nanorodNanorod
In nanotechnology, nanorods are one morphology of nanoscale objects. Each of their dimensions range from 1–100 nm. They may be synthesized from metals or semiconducting materials. Standard aspect ratios are 3-5. Nanorods are produced by direct chemical synthesis...
s were synthesized as polymers in two modifications.
The red-brown phase differs from red phosphorus and is also stable in air for weeks. Electron microscope
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
showed the red-brown form as having long, parallel nanorods with a diameter between 0.34 nm
Nanometre
A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter...
and 0.47 nm.
| Form | white(α) | white(β) | violet | black |
|---|---|---|---|---|
| Symmetry | Body-centred cubic Cubic crystal system In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.... |
Triclinic | Monoclinic | Orthorhombic |
| Pearson symbol Pearson symbol The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8... |
aP24 | mP84 | oS8 | |
| Space group Space group In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct... |
I3m | P No.2 | P2/c No.13 | Cmca No.64 |
| Density Density The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight... (g/cm3) |
1.828 | 1.88 | 2.36 | 2.69 |
| Bandgap (eV) | 2.1 | 1.5 | 0.34 | |
| Refractive index Refractive index In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium.... |
1.8244 | 2.6 | 2.4 |

